ABSTRACT
Objective: To investigate the effects of quercetin in a model of bleomycin-induced pulmonary inflammation and fibrosis. Methods: Seventynine
adult male hamsters were randomized to receive intratracheal (IT) instillations and intraperitoneal (IP) injections in four configurations:
IP vehicle/IT saline (VS group, n = 16); IP quercetin/IT saline (QS group, n = 16); IP vehicle/IT bleomycin (VB group, n = 27); and IP quercetin/
IT bleomycin (QB group, n = 20). Quercetin and bleomycin were administered at 30 mg/kg/day and 10 U/kg, respectively. Quercetin was
started/discontinued 3 days before/14 days after the IT instillations. Results: The mortality rate was significantly higher in the VB group
than in the other groups (44% vs. VS: 0%; QS: 0%; and QB: 15%). Lung levels of thiobarbituric acid reactive substances (× 10−2 nmol/mg)
were significantly higher in the VB group (6.6 ± 1.3 vs. VS: 5.5 ± 0.8; QS: 2.5 ± 0.6; and QB: 5.8 ± 0.6). Lung levels of reduced glutathione
(× 10−2 nmol/mg) were significantly lower in the VB/QB groups than in the VS/QS groups (28.9 ± 13.8/28.6 ± 14.8 vs. 43.9 ± 16.0/51.1 ± 20.3),
whereas those of hydroxyproline (mg/g) were significantly higher (201.6 ± 37.3/177.6 ± 20.3 vs. 109.6 ± 26.1/117.5 ± 32.0).Mean lung septal
thickness (μm) was greatest in the VB group (16.9 ± 3.2 vs. VS: 3.0 ± 0.3; QS: 3.3 ± 0.2; and QB: 5.2 ± 1.1). Conclusions: In a hamster model
of lung injury, quercetin exhibited anti-inflammatory effects that are related, at least in part, to its antioxidant properties.
Keywords:
Pulmonary fibrosis; Bleomycin; Flavonoids; Lipid peroxidation.
RESUMO
Objetivo: Investigar os efeitos da quercetina em um modelo de inflamação pulmonar e fibrose induzidas por bleomicina. Métodos: Setenta
e nove hamsters machos adultos foram randomizados para aplicação de injeções pelas vias intratraqueal (IT) e intraperitoneal (IP) em
quatro configurações: veículo IP/salina IT (grupo VS, n = 16); salina IT/quercetina IP (grupo QS, n = 16); bleomicina IT/veículo IP (grupo
VB, n = 27); e bleomicina IT/quercetina IP (grupo QB, n = 20). A quercetina e a bleomicina foram aplicadas em doses de 30 mg/kg/dia e
10 U/kg, respectivamente.A quercetina foi iniciada/suspensa 3 dias antes/14 dias depois das injeções IT. Resultados: A taxa de mortalidade
do grupo VB foi significantemente superior à dos demais grupos (44% vs. VS: 0%; QS: 0%; QB: 15%). O grupo VB exibiu níveis pulmonares
de substâncias reativas ao ácido tiobarbitúrico (× 10−2 nmol/mg) significativamente maiores (6,6 ± 1,3 vs. VS: 5,5 ± 0,8; QS: 2,5 ± 0,6; e
QB: 5,8 ± 0,6).Os grupos VB/QB mostraram níveis pulmonares de glutationa reduzida (× 10−2 nmol/mg) significativamente menores que
os dos grupos VS/QS (28,9 ± 13,8/28,6 ± 14,8 vs. 43,9 ± 16,0/51,1 ± 20,3) e níveis de hidroxiprolina (mg/g) significativamente maiores
(201,6 ± 37,3/177,6 ± 20,3 vs. 109,6 ± 26,1/117,5 ± 32,0). Conclusões: Em um modelo animal de lesão pulmonar, a quercetina exibiu efeitos
antiinflamatórios que são relacionados, pelo menos em parte, a suas propriedades antioxidantes.
Palavras-chave:
Fibrose pulmonar; Bleomicina; Flavonóides; Peroxidação de lipídeos.
IntroductionPulmonary fibrosis is a disorder characterized by complex inflammatory processes that result in excessive fibroblast proliferation and progressive deposition of connective tissue in the pulmonary parenchyma. Disorders of this type lead to severe deterioration of lung function, with limiting symptoms and poor quality of life. Unfortunately, despite the severity of the disease, the treatments currently available for pulmonary fibrosis provide only minimal benefits and have significant side effects.
Quercetin is a dietary flavonoid ubiquitous in nature. It is found in many plants, such as onions, broccoli, and tea. A significant number of chemical properties and pharmacological effects have been attributed to this agent.(1,2) Quercetin exhibits potent antioxidant effects, combining with free radical species to form less reactive phenoxy radicals.(3,4) Quercetin also has inhibitory effects on several proteins, such as tyrosine and serine/threonine kinases.(5) Quercetin has been shown to have anti-inflammatory properties in various in vitro and in vivo systems, in hepatic cirrhosis, and in pulmonary influenza virus infection.(6-9) Quercetin has also been reported to have an antiproliferative effect on cultures of fibroblast cells obtained from mice and from human keloids.(10,11) In addition, quercetin inhibits the proliferation and collagen synthesis of the myofibroblast rat cell line HSC-T6 and of rat-derived stellate cells.(12)
Such hepatic cells are thought to play a central role in liver fibrogenesis, in experimental models of chronic liver disease as well as in humans with chronic liver disease.
The objective of this study was to examine the possible beneficial effects of quercetin on the inflammatory and fibrotic responses induced by the introduction of bleomycin into the lungs of hamsters. The preliminary results indicate an anti-inflammatory effect, as evaluated through morphometric studies and assessment of oxidative stress.
MethodsSeventy-nine adult male hamsters (Mesocricetus
auratus) were obtained from the Central Animal Facility of the University of São Paulo at Ribeirão Preto School of Medicine. The local Animal Ethics Committee approved the research protocol, and the hamsters were handled according to the National Academy of Sciences standards.(13)
The animals were housed in collective cages and maintained on a 12/12-h light/dark cycle in a temperature-controlled environment (≈25 °C), with free access to standard rodent chow and water throughout the study.
Bleomycin sulfate (Bonar®) was obtained from Biosintética Laboratories (São Paulo, Brazil). The drug was diluted in sterile saline, and administered by intratracheal instillation at doses of 10 U/kg. Powdered quercetin dihydrate was obtained from Henrifarma (São Paulo, Brazil) and stored in a dark, dry environment at room temperature. The quercetin was diluted in saline and 0.2% Tween immediately preceding the injections.
Animals received daily intraperitoneal injections of 30 mg/kg, based on a previous study in which this dose attenuated kidney ischemia/reperfusion injury in rats.(14) Prior to bleomycin administration and lung removal, animals were anesthetized with intraperitoneal injection of 250 mg/kg of 2.5% tribromoethanol (Sigma-Aldrich, St. Louis, MO, USA). All biochemical determinations were performed using high quality, analytical grade reagents.
The hamsters were allowed to acclimate to the new environment for 3 days, after which they were randomized to one of four groups:
1) VS (n = 16): intraperitoneal vehicle and intratracheal saline
2) QS (n = 16): intraperitoneal quercetin solution and intratracheal saline
3) VB (n = 27): intraperitoneal vehicle and intratracheal bleomycin
4) QB (n = 20): intraperitoneal quercetin solution and intratracheal bleomycin
Bleomycin or saline was administered 3 days after the beginning of quercetin or vehicle treatment (Figure 1). The treatments were continued for an additional 14 days after intratracheal instillation, at which time the hamsters were killed.
The intraperitoneal injections were all given in the early morning, with the animals awake. The intratracheal instillations were performed in the early afternoon, after the hamsters had been anesthetized as described above.
A small incision was made in the neck, and the instillations were performed under direct vision of the trachea, using plastic syringes and fine needles. The surgical wound was closed with stitches, after which the head and thorax of each animal was maintained in an elevated position until recovery.
At approximately 24 h after the final injection of quercetin or vehicle, the animals were anesthetized and a tracheotomy was performed in order to remove the lungs. The animals were still breathing when a fine plastic catheter was inserted into and fixed to the trachea. Thoracotomy and laparotomy were performed, after which the animals were killed by abdominal aorta transection. The heart and lungs were removed en bloc and then dissected. The main right bronchus was ligated, after which the right lung was removed and divided into three portions. Each of those portions was frozen in liquid nitrogen and stored at -70 °C for later biochemical analysis. The left lung was inflated using the plastic catheter with buffered 10% formalin on a 20-cm column. The left main bronchus was ligated, and the entire left lung was immersed in 10% formalin for tissue fixation.
As an index of membrane lipid peroxidation, the levels of thiobarbituric acid reactive substances (TBARS) were measured in lung samples. Right lung samples were thawed, the tissue was homogenized, and the levels of TBARS were measured using colorimetric techniques.(15) The levels of reduced glutathione (GSH) in tissue homogenates were also measured using a method for quantifying acid-soluble thiol.(16) Lung tissue levels of hydroxyproline were determined by assessing collagen deposition, which was quantified using an adaptation of a classical method.(17) All optical readings were taken using a Beckman DU 640 spectrophotometer (Beckman Inc., Fullerton, CA, USA).
Left lung sections were stained with hematoxylin-eosin (H&E) and subjectively evaluated under light microscopy by a pulmonary pathologist who was blinded to the animal groups. Morphometric studies were performed using a Leica DMRB imaging system (Leica, Wetzlar, Germany) connected to a Pentium 4 IBM personal computer. The Leica Qwin software was used for image processing. Alveolar septal thickness was measured in ten 400× optical fields, as described elsewhere.(18) Thirty measurements were performed for each field, and, therefore, 300 measurements were available for each lung. For each animal, the final value of alveolar septal thickness was calculated as the mean value of the 300 measurements.
All results are expressed as mean ± standard deviation. Comparisons of the survival rates among groups were performed by applying the t-test for proportions in all combinations.(19) Comparisons of the biochemical and morphological results were performed by one-way analysis of variance followed by the Tukey's post-test, when indicated. The level of statistical significance was set at p < 0.05.(19) Statistical analyses were performed using the statistical software Sigma Stat for Windows, version 2 (Jandel, San Rafael, CA, USA).
ResultsIn order to set up four experimental groups of at least 15 animals each, it was necessary to include 79 hamsters in the study. A substantial number of premature deaths occurred in the groups that received bleomycin.
These deaths occurred at time points that were distributed over the course of the study period, without any clustering. The proportion of unexpected deaths in the VB group (12/27, 44%) was significantly higher than that observed in the other groups (VS: 0%; QS: 0%; QB: 3/20, 15%). The results shown are limited to the animals that survived until the scheduled date for euthanasia. There were no differences among the animal groups in terms of mean body weight at baseline (Table 1). The percentage variation between the final and initial total body weight [100 × (final weight - initial weight)/(initial weight)] was significantly higher in the VS and QS groups than in the VB and QB groups (Table 1).
Although lung tissue levels of TBARS were significantly higher in the VB group than in all other groups (Table 2), they were also significantly higher in the QB group than in the QS group and significantly lower in the QS group than in the VS group.
Lung levels of reduced GSH were significantly lower in the VB and QB groups than in the VS and QS groups (Table 2). However, reduced GSH levels were found to be comparable between the VS and QS groups, as well as between the VB and QB groups.
Biochemical lung tissue measurements and mean septal alveolar thickness are shown in Table 2. Due to the small amount of tissue available for biochemical analyses in some animals, it was not possible to measure the lung levels of hydroxyproline for all hamsters. Nevertheless, lung tissue levels of hydroxyproline were significantly higher in the VB and
QB groups than in the VS and QS groups. Although the mean hydroxyproline level was lower in the QB group than in the VB group, this difference did not reach statistical significance.
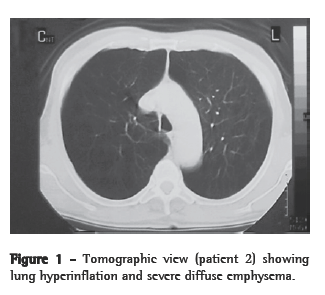
Histological studies were performed on representative left lungs, five obtained from VS group animals and eighteen obtained from animals in the QS, VB, and QB groups (six per group). The VS group lungs had a normal appearance, as did the QS group lungs (Figure 2). However, the lungs of animals receiving bleomycin only (VB group) presented significant histological changes, predominantly in a peribronchial distribution. Large areas of mixed cellular infiltrates, consisting predominantly of neutrophils were seen, accompanied by evidence of type II pneumocyte proliferation and a few hyaline membranes. Extracellular matrix deposition, accompanied by proliferation of cells with fibroblastic features, was also observed. Animals in the QB group, which received quercetin prior to administration of the bleomycin, presented significantly less bleomycin-induced inflammatory infiltrates and extracellular matrix accumulation in comparison with those in the VB group, which received only vehicle prior to bleomycin administration. Mean alveolar septal thickness was significantly greater in the VB group than in all other groups (Table 2).
 Discussion
DiscussionThe results of the present study show that quercetin attenuates the pulmonary injury induced by intratracheal administration of bleomycin. This effect was characterized by a reduction in oxidative stress and in the cellular infiltration of alveolar septal walls.
Bleomycin-induced lung injury has been extensively used as an experimental model for investigating the development of pulmonary fibrosis. Bleomycin affects DNA, producing double-strand breaks in an (oxygen and metal) ion-dependent process. Once established, the resulting interstitial pneumonia bears more resemblance to fibrotic non-specific interstitial pneumonia than to human idiopathic pulmonary fibrosis or acute lung injury.(20-22)
The animals were killed 15 days after the intratracheal instillations in order to evaluate the process at a stage in which inflammatory and proliferative phenomena were both present in the animals receiving bleomycin.
However, the markedly greater numbers of cellular infiltrates and hyaline membranes indicates that the approach adopted in this study depicts the typical profile of an acute phase of the lung injury. The use of bleomycin alone (VB group) was associated with a significant loss of total body weight and with a significant degree of (unexpected) mortality compared with controls (VS group). Administration of bleomycin alone (VB group) led to significantly higher mean lung levels of TBARS, as well as to significantly lower mean levels of reduced GSH, compared with controls (VS group). Mean septal thickness was also significantly greater in the VB group than in the VS group. These data confirm those of previous published results indicating that the bleomycin-induced inflammatory process involves increases in lung lipid peroxidation and simultaneous depletion of antioxidants at a high rate.(21,22) Indeed, various biochemical abnormalities that reflect increases in oxidative stress have also been described in clinical settings.(23,24) Mean lung tissue levels of hydroxyproline were significantly higher in the VB group than in the VS group (controls). The last is also an expected finding, since previous studies in hamsters have shown that increases in lung tissue levels of hydroxyproline can occur as early as 11 days after intratracheal administration of bleomycin.(21)
Among other pharmacological effects, quercetin prevents lipid peroxidation and scavenges superoxide radicals.(1,25,26) These effects are due to the presence of several aromatic rings containing hydroxyl radicals. On the basis of these biochemical properties, it is not surprising that, in the present study, the simultaneous use of quercetin led to significant attenuation of the bleomycin-induced increases in the levels of TBARS.
Similar effects of quercetin on tissue levels of TBARS have been described in other animal models of organ injury.(6,7,14) In the present study, the levels of TBARS were also significantly lower in the QS group than in the VS group, strongly supporting a role for quercetin as an inhibitor of lipid peroxidation even under physiological conditions.
The fact that lung levels of reduced GSH were significantly lower in the VB and QB groups than in the VS and QS groups can be explained by the consumption of reduced GSH during the activation of antioxidant mechanisms in response to bleomycin injury, as previously reported.(21)
Since the mean reduced GSH values were comparable in the VB and QB groups, we can propose that the beneficial effects of quercetin in the present model of pulmonary inflammation did not involve the GSH-dependent antioxidant system. In vitro studies of enzymatic and nonenzymatic lipid peroxidation of murine hepatic membranes support this hypothesis.(23) The concentrations of quercetin necessary for the development of anti-peroxidation effects have been shown to be considerably lower than those necessary to affect the function of enzymes related to the metabolism of reduced GSH.(24,27)
Mean septal thickness was significantly less in the QB group than in the VB group, although the morphometric analysis used in our study did not provide a clear definition of the histological components responsible for this difference. Nevertheless, the subjective examination of the stained material by a specially trained pathologist suggested that the effect was more evident on inflammatory infiltrates than on extracellular matrix accumulation. This indicates that quercetin has a significant anti-inflammatory effect on the reactive processes elicited by bleomycin.
Comparable results have been obtained in a murine model of pulmonary influenza infection, in which the use of quercetin led to significant reductions in epithelial damage and leukocyte influx.(8)
Bleomycin-induced weight loss was not influenced by quercetin injection. In fact, the degree of weight gain was significantly lower in the QS group than in the VS group. We believe that the latter finding might be explained by the potential anticholinergic effects of quercetin on the digestive tract, which would impair digestive tract motility and glandular secretions.(28)
Most importantly, the mortality rate was unexpectedly high in the QB group (15%), albeit significantly lower than that seen in the VB group (44%). We can speculate that this result was due to improvements in respiratory physiology and blood gas exchanges associated with a less inflamed lung. In addition, as described in other situations, the use of quercetin might also have decreased the amount of circulating pro-oxidative and inflammatory mediators released by the lungs, or attenuated their systemic effects.(9) Regardless of the mechanisms responsible, the reduction in the number of deaths associated with quercetin use is a supporting finding for the future development of clinical studies using flavonoid agents in patients with inflammatory or fibrotic pulmonary disorders.
Lung tissue levels of hydroxyproline in the VB group did not differ significantly from those seen in the QB group, although the mean value for the latter was somewhat lower than that obtained for the former. Initially, we can propose that, although quercetin decreased the inflammatory response, it did not greatly affect the fibrotic process itself. A recent hypothesis draws a distinction between fibrotic responses and inflammatory processes in patients with idiopathic pulmonary fibrosis/usual interstitial pneumonia.(29) However, previous experimental studies have shown that the degree of chronic fibrotic reaction to bleomycin is related to the intensity of the acute inflammatory response.(30) In addition, the antiproliferative effects of quercetin on fibroblastic function, as well as its antifibrotic effects, are known.(10,11) The findings of the present study should be interpreted with caution, since the sample size might have been too small to show a relevant difference between the bleomycin-treated hamsters receiving vehicle and those receiving quercetin in terms of the levels of hydroxyproline.
In conclusion, our preliminary results indicate that quercetin, although having no influence on collagen deposition, attenuates the pulmonary oxidative stress and inflammatory response resulting from intratracheal administration of bleomycin. It is of note that these findings were made in a model of acute lung injury rather than in a model of proliferative fibrotic processes. Additional studies are necessary to better characterize the mechanisms involved in this response.
References 1. Formica JV, Regelson W. Review of the biology of Quercetin and related bioflavonoids. Food Chem Toxicol. 1995;33(12):1061-80.
2. Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19-34.
3. Cai Q, Rahn RO, Zhang R. Dietary flavonoids, quercetin, luteolin and genistein, reduce oxidative DNA damage and lipid peroxidation and quench free radicals. Cancer Lett. 1997;119(1):99-107.
4. Johnson MK, Loo G. Effects of epigallocatechin gallate and quercetin on oxidative damage to cellular DNA. Mutat Res. 2000;459(3):211-8.
5. Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351(Pt 1):95-105.
6. Morikawa K, Nonaka M, Narahara M, Torii I, Kawaguchi K, Yoshikawa T, et al. Inhibitory effect of quercetin on carrageenan-induced inflammation in rats. Life Sci. 2003;74(6):709-21.
7. Pavanato A, Tuñón MJ, Sánchez-Campos S, Marroni CA, Llesuy S, González-Gallego J, et al. Effects of quercetin on liver damage in rats with carbon tetrachloride-induced cirrhosis. Dig Dis Sci. 2003;48(4):824-9.
8. Kumar P, Sharma S, Khanna M, Raj HG. Effect of Quercetin on lipid peroxidation and changes in lung morphology in experimental influenza virus infection. Int J Exp Pathol. 2003;84(3):127-33.
9. Savov VM, Galabov AS, Tantcheva LP, Mileva MM, Pavlova EL, Stoeva ES, et al. Effects of rutin and quercetin on monooxygenase activities in experimental influenza virus infection. Exp Toxicol Pathol. 2006;58(1):59-64.
10. Phan TT, Lim IJ, Sun L, Chan SY, Bay BH, Tan EK, et al. Quercetin inhibits fibronectin production by keloid-derived fibroblasts. Implication for the treatment of excessive scars. J Dermatol Sci. 2003;33(3):192-4.
11. Phan TT, Sun L, Bay BH, Chan SY, Lee ST. Dietary compounds inhibit proliferation and contraction of keloid and hypertrophic scar-derived fibroblasts in vitro: therapeutic implication for excessive scarring. J Trauma. 2003;54(6):1212-24.
12. Kang LP, Qi LH, Zhang JP, Shi N, Zhang M, Wu TM, et al. Effect of genistein and quercetin on proliferation, collagen synthesis, and type I procollagen mRNA levels of rat hepatic stellate cells. Acta Pharmacol Sin. 2001;22(9):793-6.
13. Institute of Laboratory Animal Resources (U.S.). Guide for the Care and Use of Laboratory Animals. Washington, D.C.: National Academy Press, 1996. 140 p.
14. Singh D, Chander V, Chopra K. The effect of quercetin, a bioflavonoid on ischemia/reperfusion induced renal injury in rats. Arch Med Res. 2004;35(6):484-94.
15. Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302-10.
16. Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25(1):192-205.
17. Rojkind M, González E. An improved method for determining specific radioactivities of proline-14C and hydroxyproline-14C in collagen and in noncollagenous proteins. Anal Biochem. 1974;57(1):1-7.
18. de Rezende MC, Martinez JA, Capelozzi VL, Simões MJ, Beppu OS. Protective effect of aminoguanidine in a murine model of pulmonary fibrosis induced by bleomycin. Fundam Clin Pharmacol. 2000;14(6):561-7.
19. Zar JH. Biostatistical analysis. Upper Saddle River, N.J.: Prentice Hall; 1999.
20. Hay J, Shahzeidi S, Laurent G. Mechanisms of bleomycin-induced lung damage. Arch Toxicol. 1991;65(2):81-94.
21. Giri SN, Chen ZL, Younker WR, Schiedt MJ. Effects of intratracheal administration of bleomycin on GSH-shuttle enzymes, catalase, lipid peroxidation, and collagen content in the lungs of hamsters. Toxicol Appl Pharmacol. 1983;71(1):132-41.
22. Yao HW, Zhu JP, Zhao MH, Lu Y. Losartan attenuates bleomycin-induced pulmonary fibrosis in rats. Respiration. 2006;73(2):236-42.
23. Cantin AM, North SL, Fells GA, Hubbard RC, Crystal RG. Oxidant-mediated epithelial cell injury in idiopathic pulmonary fibrosis. J Clin Invest. 1987;79(6):1665-73.
24. Cantin AM, Hubbard RC, Crystal RG. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1989;139(2):370-2.
25. Afanas'ev IB, Dorozhko AI, Brodskii AV, Kostyuk VA, Potapovitch AI. Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem Pharmacol. 1989;38(11):1763-9.
26. O'Brien NM, Woods JA, Aherne SA, O'Callaghan YC. Cytotoxicity, genotoxicity and oxidative reactions in cell-culture models: modulatory effects of phytochemicals. Biochem Soc Trans. 2000;28(2):22-6.
27. Galvez J, de la Cruz JP, Zarzuelo A, Sanchez de la Cuesta F. Flavonoid inhibition of enzymic and nonenzymic lipid peroxidation in rat liver differs from its influence on the glutathione-related enzymes. Pharmacology. 1995;51(2):127-33.
28. Morales MA, Tortoriello J, Meckes M, Paz D, Lozoya X. Calcium-antagonist effect of quercetin and its relation with the spasmolytic properties of Psidium guajava L. Arch Med Res. 1994;25(1):17-21.
29. Selman M, King TE, Pardo A; American Thoracic Society; European Respiratory Society; American College of Chest Physicians. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134(2):136-51.
30. Shen AS, Haslett C, Feldsien DC, Henson PM, Cherniack RM. The intensity of chronic lung inflammation and fibrosis after bleomycin is directly related to the severity of acute injury. Am Rev Respir Dis. 1988;137(3):564-71.
____________________________________________________________________________________________________________________
Study carried out in the Department of Clinical Medicine of the Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo -FMRP/USP, University of São Paulo at Ribeirão Preto School of Medicine - Ribeirão Preto, Brazil.
1. Associate Professor. Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo - FMRP/USP, University of São Paulo at Ribeirão Preto School of Medicine - Ribeirão Preto, Brazil.
2. Professor in the Department of Pathology. Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo - FMRP/USP, University of São Paulo at Ribeirão Preto School of Medicine - Ribeirão Preto, Brazil.
3. Assistant Technician (Biomedical Professional) in the Department of Clinical Medicine. Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo - FMRP/USP, University of São Paulo at Ribeirão Preto School of Medicine - Ribeirão Preto, Brazil.
4. Assistant Technician (Biologist) in the Department of Clinical Medicine. Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo - FMRP/USP, University of São Paulo at Ribeirão Preto School of Medicine - Ribeirão Preto, Brazil.
5. Full Professor in the Department of Clinical Medicine. Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo - FMRP/USP, University of São Paulo at Ribeirão Preto School of Medicine - Ribeirão Preto, Brazil.
Correspondence to: José Antônio Baddini Martinez. Hospital das Clínicas de Ribeirão Preto, Av. Bandeirantes, 3900, CEP 14048-900, Ribeirão Preto, SP, Brasil.
Tel 55 16 3966-6562. E-mail: jabmarti@fmrp.usp.br
Submitted: 21 June 2007. Accepted, after review: 15 October 2007.
**A versão completa em português deste artigo está disponível em www.jornaldepneumologia.com.br





