ABSTRACT
Amyloidosis is a disease characterized by extracellular deposition of fibrillar protein in organs and tissues. Primary tracheal amyloidosis is rare. We report here a case of a 55-year-old man with tracheal amyloidosis hospitalized for acute respiratory insufficiency and with a history of recent episodes of pneumonia. Chest X-ray and chest computed tomography showed tracheal obstruction due to a tumor. A passage was created in order to relieve the symptoms. Histological examination (Congo red staining) revealed amyloid deposits but no evidence of neoplasia. Although this is a rare clinical condition, its importance is discussed regarding the differential diagnosis of tracheal tumors and the repercussions for therapeutic decision-making.
Keywords:
Amyloidosis; Respiratory insufficiency; Congo red; Airway obstruction.
RESUMO
A amiloidose é uma doença caracterizada pelo depósito extracelular de proteínas fibrilares em órgãos e tecidos. A forma traqueal primária isolada é rara. Relata-se o caso de um homem, 55 anos de idade, portador de amiloidose traqueal que interna por insuficiência respiratória aguda, com história de pneumonias prévias recentes. Radiograma de tórax seguido de tomografia computadorizada de tórax revelou obstrução da traquéia por tumoração. Foi realizada tunelização para alívio temporário dos sintomas. Os achado histológicos não revelaram neoplasia, mas identificaram substância amilóide pelo vermelho congo. Embora a baixa freqüência desta situação clínica, é discutida sua importância no diagnóstico diferencial de tumores de traquéia e a repercussão na conduta terapêutica
Palavras-chave:
Amiloidose; Insuficiência respiratória; Vermelho congo; Obstrução das vias respiratórias.
IntroductionAmyloidosis is defined as an idiopathic systemic disorder characterized by the deposition of fibrillary and insoluble proteins in organs or tissues and is considered a highly uncommon clinical condition.(1-3) The term amyloid was coined by Virchow in the mid-19th century in autopsy studies, referring to the staining proprieties of deposits in the hepatic tissue of a substance similar to starch, soon after the application of iodine and sulfuric acid. The disease becomes clinically relevant when, in its diffuse form, it affects the function of organs, or when, in its localized form, it causes the mass effect (amyloidoma).(4)
Most cases are classified as primary or secondary, although the World Health Organization still defines other groups and subgroups, all based in the type and structure of the precursor protein component.(5)
In the chest, the cardiac form is the most frequently observed,(4) although the respiratory impairment was first described in 1877 by Lesser.(3) The primary pulmonary form can occur as tracheobronchial (nodular or diffuse) and parenchymatous (alveolar septal or nodular).(1,3,6)
Although rare, tracheobronchial involvement is the most common of the pulmonary forms.(1,3,6) In a review of cases with tracheal involvement carried out in 1983, only 67 cases had been reported in the international literature, 57 cases being of diffuse infiltrating amyloidosis and the remaining cases being of the tumor-nodular form.(3) In a study of autopsies carried out in 223 patients with amyloidosis,(7) 68 (31%) had pulmonary involvement, 23 with the primary form.
Here, a case of primary amyloidosis in the tracheobronchial form is presented with the objective of reminding physicians of the existence of a tumor that, albeit rare, should be included in the differential diagnosis of tracheal obstruction, so that the best therapy can be chosen.
Case reportA 55-year-old male, a former smoker (20 pack-years) who quit smoking 15 years prior, sought medical attention at the Emergency Room of the Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS, Pontifical Catholic University of Rio Grande do Sul) São Lucas Hospital. Through remote anamnesis, it was known that he had experienced progressive dyspnea upon exertion for the six preceding months, for which he had been hospitalized on two occasions in other centers due to pneumonia, both times in the left lower lobe, which had resolved satisfactorily with antibiotics. Although it was not possible to access the relevant X-rays, there was no clinical-radiological suspicion of obstruction of the airways based on the information provided by the patient. The patient had been on propanolol for systemic arterial hypertension for several months. The physical examination, performed in the emergency room, revealed that the patient presented good general health status, with a respiratory rate of 36 breaths/min, although there was intense intercostal retraction and the patient was in curled position. Pulmonary auscultation revealed reduced breath sounds on the left and mild bilateral wheezing with no stridor. At the time, oxygen saturation was 89% on room air.
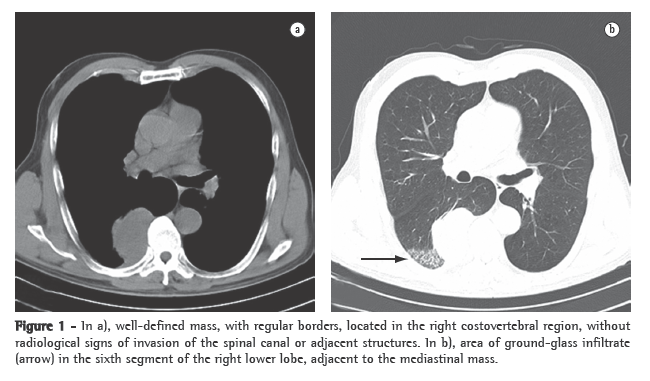
A chest X-ray showed evidence of atelectasis in the left lower lobe of the lung, with elevated hemidiaphragm on the same side, deviation of the trachea and a possible lesion in the trachea. Due to the severity of the clinical status, the patient was hospitalized after the first measures for clinical stabilization had been taken in the emergency room. Fiberoptic bronchoscopy revealed an extensive mobile mass, with near-total obstruction, in the inferior third of the trachea, at the emergence of the left main bronchus (Figure 1). A computed tomography scan of the chest confirmed the presence of a tumor obstructing the trachea (Figure 2). Rigid bronchoscopy was indicated in order to puncture and aspirate the tumor, a procedure that effectively removed 90% of the lesion. Following this procedure, the patient presented relevant improvement of the dyspnea and the cough.

The histopathological examination of the material revealed characteristic, acellular amyloid material, which was revealed by histochemical staining with Congo red (Figure 3). New fiberoptic bronchoscopy revealed residual lesion in the left main bronchus. The patient was referred to surgical removal of the remaining lesion due to the possibility of tumor recurrence at this stump. Segmental resection of the left tracheobronchial angle with primary bronchial anastomosis was performed. The postoperative period progressed with no complications. Later bronchoscopic examination revealed cicatrization of the anastomosis, with no stenosis or granuloma. The patient presented significant clinical improvement, with resolution of the symptoms. At this writing, the patient was under follow-up treatment as an outpatient clinic at our hospital, with evaluations every six months and no evidence of recurrence to date.
 Discussion
DiscussionWith the current report, we aim to remind clinicians, radiologists and thoracic surgeons of a clinical situation which, albeit rare, should be included in the differential diagnosis of tracheal obstruction. The patient described here had presented symptoms and signs for six months. Those complaints were not given adequate weight until when, already in pronounced respiratory insufficiency, he sought treatment in the emergency room of our hospital.
Tracheobronchial amyloidosis is considered a specific type of amyloidosis with deposition of fibrillary protein in the respiratory system.(1,3,8) This entity is rarely observed concomitantly in the lung parenchyma and tracheobronchial tree.(8)
The case reported here fits well into this model described for tumor lesion, the teachings of which are therefore numerous. It highlights the fact that the diagnostic possibility of obstruction in the tracheobronchial airway should be included in cases of patients with intense dyspnea and wheezing. This possibility must be especially considered in cases of recurrent pneumonia in the same location, as occurred in our patient, who had previously been evaluated only in emergency rooms.(9) Despite the fact that amyloidosis is an entity that has rarely been observed,(2,8) and that only three previous cases have been reported to have occurred in Brazil,(10-12) it must be included among the differential diagnoses of tracheal tumor.
Due to localized involvement, the physiopathology of amyloidosis suggests that there is some type of abnormal immune response by the bronchus-associated lymphoid tissue. In the trachea, amyloidosis produces diffuse patches and tumor masses, simulating neoplasms.(3) The type of amyloid deposition is different from what occurs in the systemic response.(13)
The clinical profile is typically as described here: a male patient in the fifth decade of life, presenting cough, dyspnea and occasional hemoptysis. Depending on the size and location of the lesion, pulmonary atelectasis can occur, as can obstructive pneumonia.(4) If the obstruction is located in the trachea, the physical examination will reveal bilateral wheezing.(8)
Radiological and endoscopic examinations are useful for locating the lesion and evaluating the architecture of the affected area, although the definitive diagnosis usually requires histopathological confirmation.
The histopathological diagnosis is made by the finding of amyloid, which is an inert, proteinaceous, homogeneous, acellular eosinophilic material that, when subjected to histochemical staining with Congo red, presents green birefringence under polarized light.(1,2) In tracheobronchial amyloidosis, deposits of this substance in the submucosa form irregular nodules or diffuse lamina, covered with bronchial epithelium. In the nodular form, amyloid masses are surrounded by plasma cells, lymphocytes, and giant cells. In alveolar septal amyloidosis, the amyloid is deposited between the capillary lumen and the alveolar epithelial cells.
There is no known effective, definitive treatment, although numerous forms of therapy have been tested, as has surgery, depending on many factors such as the degree of involvement, symptoms and the possibility of resection.(13-18) Laser ablation therapy, used until recently, was found to have little to no effect on the course of the disease, especially in cases of diffuse tracheobronchial amyloidosis.(13) Although the combination of prednisone and melphalan has been widely used in treating the systemic form of the disease,(8,19) there is no compelling evidence that this treatment regimen presents benefits for patients with diffuse tracheobronchial amyloidosis. Previous studies revealed benefit, in the long term, showing improvement of the symptoms in a two-year follow-up.
(15,16)
In cases of nodular-tumor involvement, in which there is mechanical obstruction, bronchoscopy or surgical resection are the treatments of choice,(20) although they are considered palliative, since they do not cure the disease or prevent recurrence.(18) In the case reported here, the possibility of recurrence led the surgical team to attempt definitive excision, at a second time point, through segmental resection of the left tracheobronchial angle and primary bronchial anastomosis.
The prognosis of amyloidosis varies according to the degree of impairment and characteristics of the patient. Cases have been reported in which the disease remained stable for long periods. Therefore, clinical and radiological observation can be an acceptable course of action.
Conversely, some cases can evolve to deterioration of pulmonary function and clinical complications, leading to death. In diffuse tracheobronchial amyloidosis, the estimated five-year survival rate is 30-50%, although treatment with radiotherapy can improve this prognosis. In the case presented here, there was no evidence of resurgence of the disease after one year of follow-up evaluation.
References 1. Capizzi SA, Betancourt E, Prakash UB. Tracheobronchial amyloidosis. Mayo Clin Proc. 2000;75(11):1148-52.
2. Falk RH, Skinner M. The systemic amyloidoses: an overview. Adv Intern Med. 2000;45:107-37.
3. Gillmore JD, Hawkins PN. Amyloidosis and the respiratory tract. Thorax. 1999 May;54(5):444-51.
4. Georgiades CS, Neyman EG, Barish MA, Fishman EK. Amyloidosis: review and CT manifestations. Radiographics. 2004;24(2):405-16.
5. Nomenclature of amyloid and amyloidosis. WHO-IUIS Nomenclature Sub-Committee. Bull World Health Organ. 1993;71(1):105-12.
6. Falk RH, Comenzo RL, Skinner M. The systemic amyloidoses. N Engl J Med. 1997;337(13):898-909.
7. Smith RR, Hutchins GM, Moore GW, Humphrey RL. Type and distribution of pulmonary parenchymal and vascular amyloid. Correlation with cardiac amyloid. Am J Med. 1979;66(1):96-104.
8. O'Regan A, Fenlon HM, Beamis JF Jr, Steele MP, Skinner M, Berk JL. Tracheobronchial amyloidosis. The Boston University experience from 1984 to 1999. Medicine (Baltimore). 2000;79(2):69-79.
9. Rekik WK, Ayadi H, Ayoub A. [Localized tracheobronchial amyloidosis: a rare cause of pseudo-asthma][Article in French]. Rev Pneumol Clin. 2001;57(4):308-10.
10. Silva LM, Bellicanta J, Marques RD, Silva LC. Tracheobronchial amyloidosis. J Bras Pneumol. 2004;30(6):581-4.
11. Schade L, Carmes ER, Barros JA. Mediastinal lymph node amyloidosis in a patient with sarcoidosis. J Bras Pneumol. 2007;33(2):222-5.
12. Montessi J, Almeida EP, Vieira JP, Horta CM, Abreu MM, Bolognani ED et al. Pulmonary amyloidosis: radiographic finding of nodular opacities in a heavy smoker. J Bras Pneumol. 2007;33(3):343-6.
13. Thompson PJ, Ryan G, Laurence BH. Laser photoradiation therapy for tracheobronchial amyloid. Aust N Z J Med. 1986;16(2):229-30.
14. Hof DG, Rasp FL. Spontaneous regression of diffuse tracheobronchial amyloidosis. Chest. 1979;76(2):237-9.
15. Kalra S, Utz JP, Edell ES, Foote RL. External-beam radiation therapy in the treatment of diffuse tracheobronchial amyloidosis. Mayo Clin Proc. 2001;76(8):853-6.
16. Kurrus JA, Hayes JK, Hoidal JR, Menendez MM, Elstad MR. Radiation therapy for tracheobronchial amyloidosis. Chest. 1998;114(5):1489-92.
17. Madden BP, Lee M, Paruchuru P. Successful treatment of endobronchial amyloidosis using Nd:YAG laser therapy as an alternative to lobectomy. Monaldi Arch Chest Dis. 2001;56(1):27-9.
18. Monroe AT, Walia R, Zlotecki RA, Jantz MA. Tracheobronchial amyloidosis: a case report of successful treatment with external beam radiation therapy. Chest. 2004;125(2):784-9.
19. Sepioło M, Skokowski J, Kamiński M. [A case of primary tracheo-bronchial amyloidosis][Article in Polish]. Pneumonol Alergol Pol. 1999;67(9-10):481-4.
20. Sipe JD, Cohen AS. Amyloidosis. In: Kasper DL, Harrison TR, editors. Harrison's principles of internal medicine. New York: McGraw-Hill, Medical Pub. Division; 2005. p. 2024-2029.
____________________________________________________________________________________________________________________
Study carried out at the São Lucas Hospital of the Pontifícia Universidade Católica do Rio Grande do Sul - PUCRS, Pontifical Catholic University of Rio Grande do Sul - Porto Alegre, Brazil.
1. Pulmonologist of the Medical Staff of the São Lucas Hospital. Pontifícia Universidade Católica do Rio Grande do Sul - PUCRS, Pontifical Catholic University of Rio Grande do Sul - Porto Alegre, Brazil.
2. Thoracic Surgeon on the Clinical Staff of the São Lucas Hospital. Pontifícia Universidade Católica do Rio Grande do Sul - PUCRS, Pontifical Catholic University of Rio Grande do Sul - Porto Alegre, Brazil.
3. Full Professor of Surgery at the Pontifícia Universidade Católica do Rio Grande do Sul - PUCRS, Pontifical Catholic University of Rio Grande do Sul - School of Medicine, Porto Alegre, Brazil.
4. Adjunct Professor of Pathology and Radiology at the Pontifícia Universidade Católica do Rio Grande do Sul - PUCRS, Pontifical Catholic University of Rio Grande do Sul - School of Medicine, Porto Alegre, Brazil.
5. Full Professor of Internal Medicine and Pulmonology at the Pontifícia Universidade Católica do Rio Grande do Sul - PUCRS, Pontifical Catholic University of Rio Grande do Sul - School of Medicine, Porto Alegre, Brazil.
Correspondence to: José Miguel Chatkin. Hospital São Lucas da PUCRS, Faculdade de Medicina, Pós Graduação, Av. Ipiranga, 6690, 3º andar, CEP 90610 000, Porto Alegre, RS, Brazil.
Tel 55 51 3336-5043. E-mail: jmchatkin@pucrs.br
Submitted: 29 May 2007. Accepted, after review: 25 September 2007.






