Renata Nakata Teixeira, Luzimar Raimundo Teixeira, Luiz Augusto Riani Costa, Milton Arruda Martins, Timothy Derick Mickleborough, Celso Ricardo Fernandes Carvalho
ABSTRACT
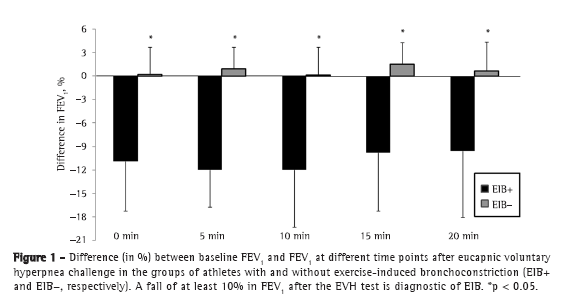
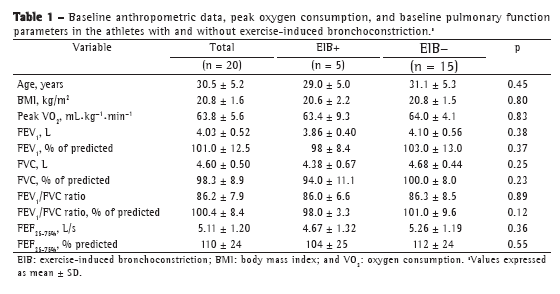
Objective: To determine the prevalence of exercise-induced bronchoconstriction among elite long-distance runners in Brazil and whether there is a difference in the training loads among athletes with and without exercise-induced bronchoconstriction. Methods: This was a cross-sectional study involving elite long-distance runners with neither current asthma symptoms nor a diagnosis of exercise-induced bronchoconstriction. All of the participants underwent eucapnic voluntary hyperpnea challenge and maximal cardiopulmonary exercise tests, as well as completing questionnaires regarding asthma symptoms and physical activity, in order to monitor their weekly training load. Results: Among the 86 male athletes recruited, participation in the study was agreed to by 20, of whom 5 (25%) were subsequently diagnosed with exercise-induced bronchoconstriction. There were no differences between the athletes with and without exercise-induced bronchoconstriction regarding anthropometric characteristics, peak oxygen consumption, baseline pulmonary function values, or reported asthma symptoms. The weekly training load was significantly lower among those with exercise-induced bronchoconstriction than among those without. Conclusions: In this sample of long-distance runners in Brazil, the prevalence of exercise-induced bronchoconstriction was high.
Keywords: Athletes; Asthma, exercise-induced; Exercise test.
RESUMO
Objetivo: Determinar a prevalência de broncoespasmo induzido por exercício em corredores brasileiros de longa distância de elite e se há uma diferença na carga de treinamento entre atletas com e sem broncoespasmo induzido por exercício. Métodos: Estudo transversal com corredores de longa distância de elite sem sintomas atuais de asma e sem diagnóstico de broncoespasmo induzido por exercício. Todos os participantes foram submetidos ao teste de hiperventilação voluntária eucápnica e ao teste cardiopulmonar de esforço máximo e responderam a questionários sobre sintomas de asma e atividade física para monitorizar sua carga de treinamento semanal. Resultados: Dos 86 atletas do sexo masculino recrutados, 20 concordaram em participar do estudo, dos quais 5 (25%) foram diagnosticados com broncoespasmo induzido por exercício. Não foram evidenciadas diferenças entre os atletas com e sem broncoespasmo induzido por exercício em relação a características antropométricas, consumo de oxigênio de pico, valores basais de função pulmonar ou sintomas de asma relatados. A carga de treinamento semanal foi significativamente menor nos atletas com broncoespasmo induzido por exercício do que naqueles sem esse diagnóstico. Conclusões: Nesta amostra de corredores de longa distância brasileiros, a prevalência de broncoespasmo induzido por exercício foi alta.
Palavras-chave: Atletas; Asma induzida por exercício; Teste de esforço.
Introduction