Denise Rossato Silva, Diego Millán Menegotto, Luis Fernando Schulz, Marcelo Basso Gazzana, Paulo de Tarso Roth Dalcin
ABSTRACT
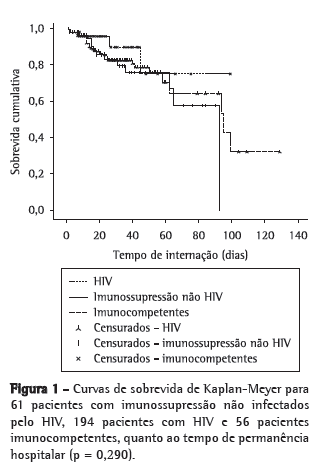
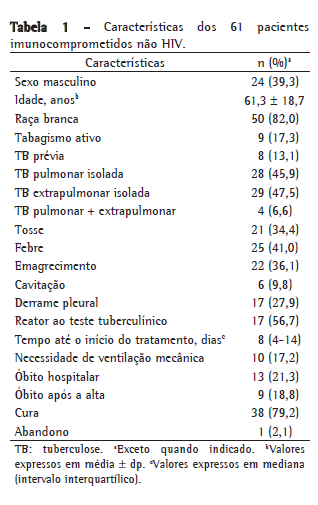
Objective: To investigate the characteristics of and risk factors for mortality among non-HIV-infected immunocompromised patients with an in-hospital diagnosis of tuberculosis. Methods: This was a two-year, retrospective cohort study of patients with an in-hospital diagnosis of tuberculosis. The predictive factors for mortality were evaluated. Results: During the study period, 337 hospitalized patients were diagnosed with tuberculosis, and 61 of those patients presented with immunosuppression that was unrelated to HIV infection. Extrapulmonary tuberculosis was found in 47.5% of cases. In the latter group, the in-hospital mortality rate was 21.3%, and the mortality rate after discharge was 18.8%. One-year survival was significantly higher among the immunocompetent patients than among the HIV patients (p = 0.008) and the non-HIV-infected immunocompromised patients (p = 0.015), although there was no such difference between the two latter groups (p = 0.848). Among the non-HIV-infected immunocompromised patients, the only factor statistically associated with mortality was the need for mechanical ventilation. Among the patients over 60 years of age, fibrosis/atelectasis on chest X-rays and dyspnea were more common, whereas fever and consolidations were less common. Fever was also less common among the patients with neoplasms. The time from admission to the initiation of treatment was significant longer in patients over 60 years of age, as well as in those with diabetes and those with end-stage renal disease. Weight loss was least common in patients with diabetes and in those using corticosteroids. Conclusions: The lower prevalence of classic symptoms, the occurrence of extrapulmonary tuberculosis, the delayed initiation of treatment, and the high mortality rate reflect the diagnostic and therapeutic challenges of tuberculosis in non-HIV-infected immunocompromised patients.
Keywords: Hospitalization; Immunosuppression; Risk factors; Tuberculosis/mortality; Immunocompromised host.
RESUMO
Objetivo: Investigar as características de pacientes imunocomprometidos não HIV com diagnóstico intra-hospitalar de tuberculose e determinar os fatores de risco para mortalidade. Métodos: Durante um período de dois anos, foi realizado um estudo de coorte retrospectivo que incluiu os pacientes com diagnóstico de tuberculose após a internação. Os fatores preditores de mortalidade foram coletados. Resultados: Durante o período do estudo, 337 pacientes foram internados e diagnosticados com TB, e desses, 61 apresentavam imunossupressão não decorrente da infecção pelo HIV. A tuberculose extrapulmonar estava presente em 47,5% dos casos. Nesse grupo, a taxa de mortalidade intra-hospitalar foi de 21,3%, e a mortalidade após a alta foi de 18,8%. Os pacientes imunocompetentes tiveram sobrevida em um ano maior que os pacientes com HIV (p = 0,008) e que os imunocomprometidos não HIV (p = 0,015), mas não houve diferença na sobrevida entre esses dois últimos grupos (p = 0,848). Entre os pacientes imunocomprometidos não HIV, o único fator estatisticamente associado à mortalidade foi a necessidade de ventilação mecânica. Entre os maiores de 60 anos, dispneia e presença de fibrose/atelectasias na radiografia de tórax foram mais comuns, enquanto febre e consolidações foram menos frequentes nesse grupo. A febre também foi um sintoma encontrado menos comumente nos pacientes com neoplasias. O tempo até o início do tratamento foi significativamente maior nos pacientes maiores de 60 anos, nos diabéticos e nos pacientes renais crônicos. Nos pacientes diabéticos e naqueles usuários de corticosteroides, o emagrecimento foi um sintoma menos frequentemente relatado. Conclusões: A menor prevalência de sintomas clássicos, a ocorrência de tuberculose extrapulmonar, o atraso no início do tratamento e a alta taxa de mortalidade refletem o desafio diagnóstico e terapêutico da tuberculose em pacientes imunocomprometidos não HIV.
Palavras-chave: Hospitalização; Imunossupressão; Fatores de risco; Tuberculose/mortalidade; Hospedeiro imunocomprometido.
Introdução