ABSTRACT
Objective: To evaluate the impact of an aggressive treatment approach using muscle flaps or omentopexy in infections of the sternum
and anterior mediastinum following sternotomy on mortality, as compared to that of a conservative treatment approach. Methods: Data
were collected prior to, during and after the surgical procedures. Group A (n = 44) included patients submitted to conservative treatment-
debridement together with resuture or continuous irrigation with polyvinylpyrrolidone-iodine solutions, or even with second-intention
wound healing (retrospective data). Group B (n = 9) included patients in whom infection was not resolved with conservative treatment, and
who therefore underwent aggressive treatment (intermediate phase). Group C (n = 28) included patients primarily submitted to aggressive
treatment (prospective data). Results: Postoperative hospital stays were shorter in the patients submitted to aggressive treatment (p < 0.046).
There were 7 deaths in group A, 1 in group B, and 2 in group C. However, the classical level of significance of α = 0.05 was not reached.
Conclusion: Aggressive treatment also proved to be effective when the infection was not resolved with conservative treatment. These
findings demonstrate that the proposed treatment provides excellent results.
Keywords:
Surgical flaps; Osteomyelitis; Mediastinitis; Thoracic surgery.
RESUMO
Objetivo: Avaliar o impacto do tratamento agressivo com retalho muscular e/ou omentopexia nas infecções do esterno e mediastino anterior
em pós-operatório de esternotomia sobre a mortalidade, comparando-o ao do tratamento conservador. Métodos: Foram coletados dados
pré-, trans- e pós-operatórios. O grupo A (n = 44) incluiu pacientes submetidos ao tratamento conservador-desbridamento associado a
ressutura e/ou a irrigação contínua com solução de polivinilpirrolidona-iodo, ou ainda a cicatrização por segunda intenção (dados retrospectivos).
O grupo B (n = 9) incluiu pacientes nos quais não houve resolução da infecção com o tratamento conservador e que, por isso,
foram submetidos ao tratamento agressivo (fase intermediária). O grupo C (n = 28) incluiu pacientes submetidos primariamente ao tratamento
agressivo (dados prospectivos). Resultados: Identificou-se menor tempo de internação pós-operatória nos pacientes submetidos ao
tratamento agressivo (p < 0,046). Houve 7 óbitos no grupo A, 1 no grupo B e 2 no grupo C. Entretanto, o nível de significância clássico de
α = 0,05 não foi atingido. Conclusões: O tratamento agressivo mostrou-se também adequado para aquelas infecções em que o tratamento
conservador não foi resolutivo. Esses achados demonstram que o tratamento proposto tem excelentes resultados.
Palavras-chave:
Retalhos cirúrgicos; Osteomielite; Mediastinite; Cirurgia torácica.
IntroductionSuppurative mediastinitis following sternotomy is an extremely severe complication of open heart surgery, occurring in 0.4 to 5% of the cases.(1,2) The mean incidence in the largest, most recent reviews is approximately 1%.(2-4) A second operation for debridement and drainage, together with long-term hospitalization for antibiotic therapy and repeated surgical interventions, is the rule. Mortality is significant, reaching up to 40% in various studies.(1)
It has been postulated that mediastinal infections following sternotomy start in a limited area of the sternum, manifesting as osteomyelitis, with minimal or no external signs of infection.(5,6) Dehiscence of the sternum occurs a few days thereafter as a consequence of the infection. Some believe that sternal instability, together with disruption of the skin barrier, allows bacteria to enter the deeper layers. Others hypothesize that the pathogenesis of mediastinitis involves inadequate mediastinal drainage, which leads to the appearance of a retrosternal collection-dead space-that acts as a culture medium for bacterial growth.(2)
It is widely held that the obliteration of this retrosternal dead space is one of the most important prerequisites for the success of the surgical treatment of mediastinitis following sternotomy.(2,7-9)
Numerous retrospective and prospective studies have suggested that there are multiple risk factors for infection following median sternotomy, and there are frequently discrepancies among them. The conflicting data can be attributed primarily to the lack of uniformity in the definitions adopted, since the complications of median sternotomy range from sterile dehiscence to suppurative mediastinitis accompanied by osteomyelitis and generalized sepsis. The terms sternal osteomyelitis and mediastinitis have been mistakenly used as synonyms in an attempt to characterize deep sternal wound infection.(2,10)
Until 1963, infections following sternotomy were usually treated with debridement and open wound, although complete healing sometimes took 6 months, and, in many cases, two or more subsequent debridements were necessary.(11) High rates of severe complications, such as hemorrhage of exposed mediastinal vessels, as well as high mortality rates (up to 45%), have been reported.(12)
At the time, some authors(13) described the technique of closed irrigation of the mediastinum with antibiotic solutions via a catheter, together with debridement and sternal resuture; a technique that is still used today.(12) This technique reduced mortality to 20%, although reinterventions for resection of fistulae and areas of costochondritis were frequent.(14,15) Studies have identified excessive fungal growth, especially of the genus Candida,(16) which is why the use of dilute solutions of polyvinylpyrrolidone-iodine, which is bactericidal and fungicidal, and has low toxicity, has been recommended.
The first alternative to closed irrigation of the mediastinum was described in 1976, when transposition of the greater omentum was used in conjunction with bone and cartilage debridement, a technique with which others have also obtained favorable results.(7,8,17,18)
In 1980, one group of authors broadened this concept and reported their initial experience with muscle flaps in the treatment of infections following sternotomy-56% presented an inadequate response to treatment with closed mediastinal irrigation.(8) Morbidity was significantly reduced, and mortality decreased concomitantly with the reduction in length of hospital stay.
In the following years, numerous pedicle muscle flaps were introduced, as an alternative or in association, for the treatment of complex sternal infections, including the major pectoral, the rectus abdominis, and the dorsal muscles.(9,17-21)
Early aggressive treatment has been recommended by various authors. Early diagnosis is considered to play a key role in therapeutic success in this severe infectious complication following sternotomy.(2,9,21)
The objective of this study was to evaluate the impact that an aggressive treatment approach using muscle flaps or omentopexy in infections of the sternum and anterior mediastinum following heart surgery has on mortality, as well as its implications in terms of hospital stay, as compared to that of a conservative treatment approach. In addition, we attempted to determine the effectiveness of aggressive treatment in patients in whom the infection was not resolved with conservative treatment.
MethodsAt the Hospital de Clínicas de Porto Alegre (HCPA, Porto Alegre Hospital de Clínicas), all patients who developed infection following sternotomy in heart surgery-mediastinitis or mediastinitis accompanied by sternal osteomyelitis-between July of 1987 and July of 2000 were registered. A total of 2,648 median sternotomies were performed, and there were 81 cases of mediastinitis (3.05%).
Group A (n = 44) included patients from the retrospective phase (July of 1987 to December of 1994), in which the procedures recommended were exploratory mediastinotomy together with sternal curettage or debridement of necrotic tissue with second-intention wound healing (open wound), or sternal resuture together with continuous irrigation of the mediastinum with polyvinylpyrrolidone-iodine solution. Of those 44 patients, 12 required a second debridement.
Group B (n = 9) included patients from the initial prospective phase, in which aggressive treatment, with muscle flap or omentopexy, was performed after at least one previous intervention involving debridement and curettage.
Group C (n = 28) was characterized by aggressive primary treatment following the diagnosis of sternal infection or mediastinitis.
This study considered type 2B infections, according to the classification by El Oakley & Wright,(2) meaning clinical or microbiological evidence of infection of presternal tissues and sternal osteomyelitis, with or without mediastinal sepsis, with or without bone instability, and with infection of the retrosternal space.
The study design was approved by the Ethics in Research Committee of the HCPA, and included the following information:
1) patient data: age; gender; cardiovascular disease; and risk factors, such as chronic obstructive pulmonary disease, chronic use of immunosuppressants, history of sternotomy, obesity (prospective phase), and pyuria (prospective phase)
2) data on the heart surgery: cardiac pathology involved; antibiotic prophylaxis; length of preoperative hospital stay; surgical time; duration of extracorporeal circulation; use of the internal thoracic artery; need for intra-aortic balloon (prospective phase); need for a second operation; duration of mechanical ventilation (days); blood product transfusions (prospective phase); and external cardiac massage in the postoperative period (prospective phase)
3) data on the infection: interval between the main surgery and the diagnosis of infection; microbiological diagnosis; and accompanying infections
4) data on the treatment of the complication: surgical procedure(s) performed; antibiotics used; length of postoperative hospital stay; complications; and mortality
Mortality was defined as death directly related to sternal wound infection or death within 30 days after the reconstruction. The complications to be considered were the following: bronchopneumonia; acute kidney injury (AKI); urinary tract infection; acute respiratory distress syndrome (ARDS); complicated pleural effusion; bleeding; and areas of osteochondritis with fistulae. Seroma, hematoma, partial or complete devascularization of the flap, wound dehiscence, abdominal hernia, and abdominal wall infection were considered for groups B and C, since they are inherent to the techniques employed.
Purulent or fibrinopurulent secretion was collected for culture. Areas of necrosis or bone infection were completely resected. Frequently, when the sternum was extremely impaired, we opted for sectioning it with a saw (partial or even complete sternal resection). We systematically searched for recesses or fistulae.
Aggressive treatment involved unilateral or bilateral rotation of the major pectoral muscle, and the dislocation of the humeral segment was performed in only one side. The omentum was brought up to the chest, preferably with the two vascular pedicles preserved.
The pectoral muscle flaps were separated from the humeral insertion without a counter-incision, and vascularization was preserved through the thoracoacromial artery branches. The muscle flap was then rotated to close the sternal defect, and fixed to the rest of the viable sternum or to the intercostal muscles. The sternal borders were not reapproximated, and suction drainage tubes were installed, when necessary, to obliterate the dead space.
Omentopexy was performed through a supraumbilical midline incision, not in communication with the previous incision. The omentum was then transposed to the anterior mediastinum through a tunnel created on the anterior surface of the diaphragm muscle. It was positioned in order to fill all the dead space, including small recesses, and fixed. Fixation of the omentum to the diaphragm and aponeurosis of the rectus abdominis muscle were performed to prevent herniation.
After being discharged from the hospital, the patients underwent follow-up treatment at the outpatient clinics of the HCPA Departments of Thoracic Surgery and Cardiology, involving follow-up appointments once a week in the first month, once a month for the remainder of the first year, and once every six months thereafter.
Initially, quantitative variables were described using measures of central tendency (mean and median) and measures of dispersion (standard deviation and interquartile range). Qualitative variables were described using percentages. The intervention groups (A, B, and C) were compared in terms of these variables.
Quantitative variables were compared using analysis of variance, and differences were identified using Tukey's test. In order to compare variables with non-normal distribution, we opted for the nonparametric Kruskal-Wallis test. In order to compare qualitative variables, we used contingency tables, and significance was determined using the chi-square test.
Finally, mortality was estimated using the Kaplan-Meier survival analysis method. Significance was calculated and determined using the log-rank test.
In this study, the level of significance was set at α = 0.05. Data were processed using the Statistical Package for the Social Sciences, version 12.0 (SPSS Inc., Chicago, IL, USA).
ResultsThe proportion of males and females in the groups studied was similar (p = 0.719). The mean age, in years, was 57.9 in group A, 54.8 in group B, and 60.6 in group C, with standard deviations of 11.1, 9.7, and 10.2, respectively (p = 0.330).
The cardiovascular pathologies that resulted in surgical treatment in the groups studied are listed in Table 1. We observed a greater number of infections in surgeries due to valvular pathology in group A, but with no statistically significant differences (p = 0.144).
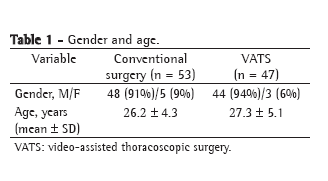
There were no statistically significant differences among the groups in terms of the following risk factors (potential confounding factors) identified in the preoperative period: diabetes (p = 0.8141); endocarditis (p = 0.828); obesity (p = 0.783); pyuria (p = 0.565); and chronic obstructive pulmonary disease (p = 0.410). The number of patients who used corticosteroids chronically was found to be greater in group C (p = 0.016).
The mean length of preoperative hospital stay was shorter in group C, although statistical analysis revealed no significant differences (p = 0.077). Surgical time ranged from 180 to 390 min in group A, from 198 to 330 min in group B, and from 240 to 360 min in group C, and was significantly longer in group A (p = 0.042). Duration of extracorporeal circulation (p = 0.692), duration of postoperative mechanical ventilation (p = 0.526), and use of the internal thoracic artery (p = 0.102) revealed no significant interference.
The interval between the surgery and the diagnosis of infection of the sternum or anterior mediastinum ranged from 3 to 26 days in group A, from 6 to 38 days in group B, and from 5 to 45 days in group C (p = 0.237). In our sample, most infections were caused by staphylococci.
Means, standard deviations, medians, and interquartile ranges related to the length of postoperative hospital stay can be seen in Table 2.
Postoperative hospital stays was shorter in group C, and this difference was statistically significant (p = 0.046).
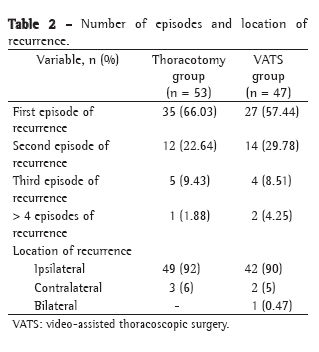
The preoperative complications in the groups studied are listed in Table 3. We observe a statistically significant decrease (p = 0.015) in the number of cases of osteomyelitis in group C. In groups B and C, there was a tendency, not significant, toward a smaller number of infectious complications, such as bronchopneumonia (p = 0.468) or urinary tract infection (p = 0.498), probably due to the fact that the absence of an irrigation device facilitates mobilization and even the return to ambulation.
There were a significantly greater number of cases of AKI in group C (p = 0.022).
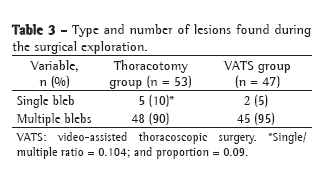
One patient in group A had hemorrhage with cardiac tamponade after debridement, and a second intervention with pericardial drainage was necessary. One patient in group B and one in group C had hematoma between the subcutaneous tissue and the muscle flap, and a second intervention was necessary to remove it (p = 0.440).
In group C, there was one case of necrosis/devascularization of the muscle flap, treated with muscle debridement together with omentopexy, and one case of devascularization of the omentum, treated with debridement together with pectoral muscle flap.
The three seromas identified after the removal of the drainage tubes were aspirated. Small dehiscences are occasionally identified at the incision site and treated conservatively by systematic changing of the dressings.
The case of diaphragmatic hernia identified in group C occurred in postoperative month 11, when, during the investigation of a pulmonary nodule, loops of small intestine were found in the anterior mediastinum.
Mortality was 15.9% (7 patients) in group A, 11.1% (one patient) in group B, and 7.14% (2 patients) in group C. There were no significant differences among the groups studied.
In group A, one death was due to ventricular fibrillation on the first day after debridement. One renal transplant patient died due to complications of chronic renal failure. The remaining 5 deaths were secondary to ARDS.
In group B, there was one death on postoperative day 21 due to cardiac arrhythmia. The death occurred on day 37 following partial resection of the sternum by omentoplasty was due to bronchopneumonia accompanied by AKI and ARDS. However, at the time of death, there was no evidence of infection in the sternum or anterior mediastinum. Of the remaining 7 patients, 2 underwent follow-up treatment for 6 months, 3 underwent follow-up treatment for 2 years, and 2 underwent follow-up treatment at another facility for one year, without presenting any complications.
In group C, there were 2 deaths due to complications (according to the definition established in this study) of heart failure and AKI. Two deaths occurred during hospitalization for treatment of the infection. However, they occurred after postoperative day 30 and were not directly related to the infection, both being due to complications of AKI (postoperative days 42 and 73). The remaining patients were evaluated in a period ranging from 2 to 5 years. Only 2 presented, as a complication, foreign body granuloma in the subcutaneous cellular tissue.
DiscussionA 3.05% incidence of sternal infections or mediastinitis is high if compared with those reported in some of the largest and most recent studies-values of approximately 1%,(6,18,22) although several others have reported incidences of up to 5%.(1) There were 10 in-hospital deaths, corresponding to a mortality rate of 12.34%, which is lower than the rates reported in most studies-from 14 to 47%.(6,22,23)
The comparison of this mortality rate with that found in group C of the present study (close to zero) reveals that this technique, though aggressive, greatly reduces mortality.(21,24-26)
Regarding risk factors, we did not intend to analyze them statistically as a cause of infection. Rather, we intended to demonstrate that risk factors are homogeneously distributed in the groups studied, so that none of them would significantly alter the number of complications and deaths in the groups studied. In other words, risk factors should be evaluated as potential confounding factors. The surgical time of the initial procedure has been implicated as a risk factor in some studies,(5) and, in the present study, it was longest in group A.
The objective of the treatment of these infections is to debride avascular and infected tissue and to obliterate dead space. After radical debridement, or even after partial or complete sternal resection, the first alternative for filling the upper two thirds of the mediastinum is the rotation of one or both major pectoral muscles. If the humeral insertion is preserved, so is innervation, perpetuating its function and avoiding atrophy. Humeral dislocation is often necessary for better mobilization, especially when the mediastinal dead space is large.(9,18,21)
The transposition of the rectus abdominis muscle requires, preferably, the existence of blood flow in the superior epigastric vessels, that is, in the ipsilateral internal thoracic artery.(9,18,19,21) In our study, we preferred to cover the lower mediastinum, when necessary, with the omentum. The use of a dorsal muscle flap seems to be indicated only in cases in which it is impossible to use the pectoral muscles, the rectus abdominis muscles, or the omentum.(9,27)
It is also of note that muscle flaps demonstrate ability to resist bacterial inoculation and control infection, since they bring an additional blood supply to an infected and poorly vascularized area, which, therefore, regardless of the stage of the infection, is equally treated. Especially impressive is their ability to resolve infections in the presence of foreign bodies, such as valves, vascular prostheses, and suture.(15,21,26)
The use of the greater omentum to fill the anterior mediastinum presents the following advantages: it is an excellent vascular supply; it is usually large; it apparently promotes angiogenesis and is resistant to infections; and it facilitates the primary closure of superficial tissues, with excellent aesthetic results.(7,17,28-30) Among the disadvantages, we can mention the possible contamination of the peritoneal cavity, the potential for the onset of epigastric hernia, and the lesser stabilization of the anterior chest wall in comparison with that obtained using muscle flaps. In addition, omentoplasty serves as an exceptional alternative in cases of muscle flap treatment failure.(9,17,24,30)
In cases of complete sternal resection, it is important to remove the adjacent costal cartilages if chondritis or signs of necrosis appear. Cartilages constitute one of the principal sites of reinfection. However, cartilage resection substantially increases the dead space, which demands that major surgery be performed to fill it. In these situations, reconstructions using a variety of muscle flaps, sometimes together with omentoplasty, have been described.(8,9,27,29,30) In the present study, major pectoral muscle flaps were used in conjunction with omentopexy in 10 of the patients in groups B and C.
In the immediate postoperative period, there was no evidence of ventilatory failure of mechanical origin secondary to chest wall instability resulting from this treatment.(18,19,21)
In the present study, the mean length of postoperative hospital stay decreased from 40.1 days (conservative treatment) to 27.4 days (aggressive treatment), which resulted in early rehabilitation and lower hospital costs. This datum is comparable to those reported in other studies.(17,18,21,27,30)
The complications found in our study are those inherent to the treatment performed. In group A, in which the treatment was debridement together with second-intention granulation or continuous irrigation, we identified 6 cases of chronic osteomyelitis with fistulae in which one or more debridements were necessary to resolve the problem. This seems to be a direct consequence of bone exposure and suggests that some alternative must be found in order to resolve the primary infection.
It is of note that the mean postoperative hospital stay after the diagnosis of infection decreased from 40.1 days (conservative treatment) to 27.4 days (aggressive treatment). This difference was statistically significant (p = 0.046).
The most important finding in this study seem to be related to mortality secondary to aggressive treatment with muscle flap or omentopexy in infections of the sternum or anterior mediastinum. The mortality found in groups B (11.1%) and C (7.14%) was lower than that found in group A (15.9%). However, the classical level of significance of α = 0.05 was not reached.
Although this study was not randomized, which represents data bias, this does not invalidate the results listed.
References 1. Newman LS, Szczukowski LC, Bain RP, Perlino CA. Suppurative mediastinitis after open heart surgery. A case control study of risk factors. Chest. 1988;94(3):546-53.
2. El Oakley RM, Wright JE. Postoperative mediastinitis: classification and management. Ann Thorac Surg. 1996;61(3):1030-6.
3. Hazelrigg SR, Wellons HA Jr, Schneider JA, Kolm P. Wound complications after median sternotomy. Relationship to internal mammary grafting. J Thorac Cardiovasc Surg. 1989;98(6):1096-9.
4. Molina JE. Primary closure for infected dehiscence of the sternum. Ann Thorac Surg. 1993;55(2):459-63.
5. Culliford AT, Cunningham JN Jr, Zeff RH, Isom OW, Teiko P, Spencer FC. Sternal and costochondral infections following open-heart surgery. A review of 2,594 cases. J Thorac Cardiovasc Surg. 1976;72(5):714-26.
6. Grossi EA, Culliford AT, Krieger KH, Kloth D, Press R, Baumann FG, et al. A survey of 77 major infectious complications of median sternotomy: a review of 7,949 consecutive operative procedures. Ann Thorac Surg. 1985;40(3):214-23.
7. Lee AB Jr, Schimert G, Shaktin S, Seigel JH. Total excision of the sternum and thoracic pedicle transposition of the greater omentum; useful strategems in managing severe mediastinal infection following open heart surgery. Surgery. 1976;80(4):433-6.
8. Jurkiewicz MJ, Bostwick J 3rd, Hester TR, Bishop JB, Craver J. Infected median sternotomy wound. Successful treatment by muscle flaps. Ann Surg. 1980;191(6):738-44.
9. Pairolero PC, Arnold PG. Management of infected median sternotomy wounds. Ann Thorac Surg. 1986;42(1):1-2.
10. Ulicny KS Jr, Hiratzka LF. The risk factors of median sternotomy infection: a current review. J Card Surg. 1991;6(2):338-51.
11. Grmoljez PF, Barner HH, Willman VL, Kaiser GC. Major complications of median sternotomy. Am J Surg. 1975;130(6):679-81.
12. Sarr MG, Gott VL, Townsend TR. Mediastinal infection after cardiac surgery. Ann Thorac Surg. 1984;38(4):415-23.
13. Shumacker HB Jr, Mandelbaum I. Continuous antibiotic irrigation in the treatment of infection. Arch Surg. 1963;86:384-7.
14. Acinapura AJ, Godfrey N, Romita M, Cunningham J Jr, Adams PX, Jacobowitz IJ, et al. Surgical management of infected median sternotomy: closed irrigation vs. muscle flaps. J Cardiovasc Surg (Torino). 1985;26(5):443-6.
15. Scully HE, Leclerc Y, Martin RD, Tong CP, Goldman BS, Weisel RD, et al. Comparison between antibiotic irrigation and mobilization of pectoral muscle flaps in treatment of deep sternal infections. J Thorac Cardiovasc Surg. 1985;90(4):523-31.
16. Glower DD, Douglas JM Jr, Gaynor JW, Jones RN, Oldham HN Jr. Candida mediastinitis after a cardiac operation. Ann Thorac Surg. 1990;49(1):157-63.
17. Heath BJ, Bagnato VJ. Poststernotomy mediastinitis treated by omental transfer without postoperative irrigation or drainage. J Thorac Cardiovasc Surg. 1987;94(3):355-60.
18. Nahai F, Rand RP, Hester TR, Bostwick J 3rd, Jurkiewicz MJ. Primary treatment of the infected sternotomy wound with muscle flaps: a review of 211 consecutive cases. Plast Reconstr Surg. 1989;84(3):434-41.
19. Neale HW, Kreilein JG, Schreiber JT, Gregory RO. Complete sternectomy for chronic osteomyelitis with reconstruction using a rectus abdominis myocutaneous island flap. Ann Plast Surg. 1981;6(4):305-14.
20. Majure JA, Albin RE, O'Donnell RS, Arganese TJ. Reconstruction of the infected median sternotomy wound. Ann Thorac Surg. 1986;42(1):9-12.
21. Jeevanandam V, Smith CR, Rose EA, Malm JR, Hugo NE. Single-stage management of sternal wound infections. J Thorac Cardiovasc Surg. 1990;99(2):256-62; discussion 262-3.
22. Ottino G, De Paulis R, Pansini S, Rocca G, Tallone MV, Comoglio C, et al. Major sternal wound infection after open-heart surgery: a multivariate analysis of risk factors in 2,579 consecutive operative procedures. Ann Thorac Surg. 1987;44(2):173-9.
23. Ivert T, Lindblom D, Sahni J, Eldh J. Management of deep sternal wound infection after cardiac surgery--Hanuman syndrome. Scand J Thorac Cardiovasc Surg. 1991;25(2):111-7.
24. Belcher P, McLean N, Breach N, Paneth M. Omental transfer in acute and chronic sternotomy wound breakdown. Thorac Cardiovasc Surg. 1990;38(3):186-91.
25. Ringelman PR, Vander Kolk CA, Cameron D, Baumgartner WA, Manson PN. Long-term results of flap reconstruction in median sternotomy wound infections. Plast Reconstr Surg. 1994;93(6):1208-14; discussion 1215-6.
26. Eckstein FS, Albes JM, Jurmann MJ, Scheule AM, Raygrotzki S, Laniado M, et al. Surgical management of persistent mediastinitis after coronary bypass grafting. Ann Thorac Surg. 1997;64(3):854-6.
27. Jones G, Jurkiewicz MJ, Bostwick J, Wood R, Bried JT, Culbertson J, et al. Management of the infected median sternotomy wound with muscle flaps. The Emory 20-year experience. Ann Surg. 1997;225(6):766-76; discussion 776-8.
28. Schroeyers P, Wellens F, Degrieck I, De Geest R, Van Praet F, Vermeulen Y, et al. Aggressive primary treatment for poststernotomy acute mediastinitis: our experience with omental- and muscle flaps surgery. Eur J Cardiothorac Surg. 2001;20(4):743-6.
29. Yoshida K, Ohshima H, Murakami F, Tomida Y, Matsuura A, Hibi M, et al. Omental transfer as a method of preventing residual persistent subcutaneous infection after mediastinitis. Ann Thorac Surg. 1997;63(3):858-9; discussion 859-60.
30. Francel TJ, Kouchoukos NT. A rational approach to wound difficulties after sternotomy: reconstruction and long-term results. Ann Thorac Surg. 2001;72(4):1419-29.
Study carried out in the Department of Thoracic Surgery of the Porto Alegre Hospital de Clínicas, Porto Alegre, Brazil.
1. Thoracic Surgeon at the Porto Alegre Hospital de Clínicas, Porto Alegre, Brazil.
2. Adjunct Professor in the Department of Thoracic Surgery. Federal University of Rio Grande do Sul, Porto Alegre, Brazil.
3. Head of the Department of Cardiac Surgery. Porto Alegre Hospital de Clínicas, Porto Alegre, Brazil.
Correspondence to: Alexandre Heitor Moreschi. Rua Mostardeiro, 265, conjunto 303, CEP 90430-001, Porto Alegre, RS, Brasil.
Tel 55 51 3222-6956. Fax 55 51 2101-8684. E-mail: moreschi_rs@terra.com.br
Financial support: None.
Submitted: 20 September 2007. Accepted, after review: 17 December 2007.




