ABSTRACT
Objective: Asthma and respiratory symptoms are common in children, and many studies have shown associations between childhood
symptoms and impaired lung function in adult life. The aim of the present study was to investigate the association of various respiratory
symptoms with wheezing patterns (persistent, early, and late-onset) and lung function, as well as to determine whether lung function was
associated with atopy or with demographic, socioeconomic, environmental, and gestational factors, in a birth cohort at 6-7 years of age.
Methods: The target population consisted of children aged 6-7 years from a birth cohort of 5,304 children born in southern Brazil in 1993.
For this follow-up evaluation, 532 of those children were randomly selected, and a sub-sample was submitted to spirometry and skin prick
tests. A questionnaire was administered to the parent(s) or legal guardian(s) of each child. Results: Spirometric values were lower in the
children with respiratory symptoms or asthma. Mean forced expiratory volume in one second/forced vital capacity ratio (FEV1/FVC ratio)
was lower in children with any of the following: current wheezing and asthma; asthma ever; four or more episodes of wheezing within the
preceding 12 months; sleep disturbance due to wheezing; and exercise-induced wheezing. Persistent wheezing was associated with lower
FEV1/FVC ratio. After multiple linear regression, exercise-induced wheezing was also associated with reduced FEV1/FVC ratio. Nonwhite skin
color and wheezing severe enough to limit speech were associated with lower FEV1. Conclusions: Children with persistent wheezing and
symptoms of severe asthma have impaired lung function at 6-7 years of age.
Keywords:
Asthma; Respiratory function tests; Respiratory sounds; Signs and symptoms, respiratory.
RESUMO
Objetivo: Asma e sintomas respiratórios são comuns na infância, e vários estudos têm demonstrado sua associação com função pulmonar
prejudicada na vida adulta. O objetivo deste estudo foi investigar a associação de diversos sintomas respiratórios com padrões de sibilância
(persistente, precoce e de início tardio) e função pulmonar aos 6-7 anos de idade em uma coorte de nascimentos, além de determinar se a
função pulmonar estava associada à atopia ou a fatores demográficos, socioeconômicos, ambientais e gestacionais. Métodos: A populaçãoalvo
compreendeu crianças de 6 a 7 anos de idade pertencentes à coorte de 5.304 nascimentos ocorridos em 1993 em Pelotas, no Sul do
Brasil. Para esse acompanhamento selecionaram-se aleatoriamente 532 dessas crianças, e uma subamostra foi submetida a espirometria
e testes cutâneos de puntura. Aplicou-se um questionário aos pais das crianças ou seus responsáveis. Resultados: Observamos valores
espirométricos mais baixos nas crianças com sintomas respiratórios e asma. A média da relação volume expiratório forçado no primeiro
segundo/capacidade
vital forçada (relação VEF1/CVF) foi menor nas crianças com sibilância atual e asma, asma alguma vez na vida, quatro
ou mais episódios de sibilância nos últimos 12 meses, sono perturbado pela sibilância e sibilância após exercícios. Sibilância persistente
foi associada a redução da relação VEF1/CVF. Após regressão linear múltipla, sibilância após exercícios também foi associada a redução da
relação VEF1/CVF. Cor da pele não-branca e fala prejudicada pela sibilância foram associadas a redução do VEF1. Conclusões: Crianças com
sibilância persistente e sintomas de asma grave apresentaram função pulmonar prejudicada aos 6-7 anos de idade.
Palavras-chave:
Asma; Testes de função respiratória; Sons respiratórios; Sinais e sintomas respiratórios
IntroductionThe prevalence of asthma in childhood is increasing worldwide, and studies suggest that childhood asthma persists into adult life in 26% to 78% of patients.(1-3)
In Brazil, there have been few population-based studies of asthma in childhood. In the Brazilian cities involved in the International Study of Asthma and Allergy in Childhood (ISAAC) study, the cumulative prevalence of physician-diagnosed asthma in 6-7 year olds ranged from 4.5% to 20.4%.(4,5) Using the same methodology, other authors found a 27% prevalence of asthma among 13-14 year olds in another Brazilian city.(6)
In a study conducted in southern Brazil, the prevalence of emergency room visits due to asthma was found to he high (31%) among preschool children, and asthma severity was found to be one of the predictors of such visits.(7)
Asthma and respiratory symptoms are quite common in children, and many studies have shown associations between respiratory symptoms in childhood and decreased lung function in adult life. In addition, a number of authors have shown that impaired lung function in childhood is a risk factor for abnormal lung function in adult life, concluding that the prognosis for asthma or respiratory symptoms in children is unfavorable.(8,9) Two longitudinal studies carried out in Australia also showed that impaired lung function in childhood can be associated with persistence of asthma in adult life.(3,10)
Appropriate evaluation of lung function and treatment of childhood asthma are important for the secondary prevention of decreased lung function and chronic pulmonary disease in adulthood.(8,9)
Few studies conducted in developing countries have investigated lung function in children with asthma symptoms, and little is known about the risk factors for asthma in such countries.
In the present study, we attempted to determine whether various respiratory symptoms were associated with lung function, as well as whether lung function was associated with atopy or with demographic, socioeconomic, environmental, and gestational factors, in a birth cohort at 6-7 years of age.
MethodsThis study was carried out in Pelotas, a city of 300,000 inhabitants in southern Brazil. The target population consisted of children aged 6-7 years from a birth cohort of 5,304 children born in 1993. For this follow-up evaluation, we selected 532 children, which corresponded to a systematic sample of 10% of the initial birth cohort, plus all low birth weight children. These children were examined at birth and at ages 6 months, 12 months, and 4 years, as described elsewhere.(7) A questionnaire was administered to the parent(s) or legal guardian(s) of each child. The evaluation included a questionnaire, spirometry, and a skin prick test. The study had a statistical power of 80% (alpha = 5%) to detect a relative risk of
2 for exposures affecting 7.5% or more of the children.
Of the 494 children who completed the questionnaire (93% of the initial sample), 148 (30%) were selected at random and invited to undergo spirometry and a skin prick test. These tests were performed in 143 (96.6%) of those 148 children.
Trained interviewers administered questionnaires to the mothers of the 494 children between June and September of 2000. Questions regarding asthma symptoms were based on the ISAAC questionnaire.(11) Data were collected regarding the prevalence of cumulative wheezing (wheezing ever) and wheezing within the preceding 12 months (current wheezing), as well as of nocturnal cough, exercise-induced wheezing, sleep disturbance due to wheezing, wheezing severe enough to limit speech, and physician-diagnosed asthma (asthma ever and current asthma).
The biological characteristics studied were gender and skin color (self-reported by the mother). The socioeconomic factors evaluated were maternal education (years of schooling) and family income (no. of times the minimum wage: R$ 130 = US$75). The gestational factors studied included gestational age, maternal smoking during pregnancy, and low birth weight. Bronchiolitis in the first year of life and smoking in the home were also investigated. Data related to gestational factors were collected at birth (in 1993). Data related to the other variables were collected in the 1998 and 2000 follow-up evaluations.
Variables related to wheezing patterns, as described by other authors,(12) included transient wheezing (wheezing from age 6 months to 4 years and not at age 6 years), persistent wheezing (wheezing from age 6 months to 6 years), and late-onset wheezing (wheezing only at age 6 years), and were evaluated after one, four, and six years of follow-up.
Baseline spirometry was performed in the Lung Function Laboratory of the Federal University of Pelotas University Hospital, and all spirometry exams were performed by a trained assistant. Forced expiratory volume in one second (FEV1), forced vital capacity (FVC), and FEV1/FVC ratio, as well as forced expiratory flow at 25%, 50%, 75%, and between 25 and 75% of FVC (FEF25%, FEF50%, FEF75%, and FEF25-75%, respectively), were measured with a pneumotachograph-based spirometer (Master Scope; Jaeger, Würtzburg, Germany), thereby meeting the American Thoracic Society (ATS) performance criteria.(13)
All forced expiratory maneuvers were performed with the children seated, wearing a nose-clip, and using a plastic mouthpiece. All measurements were made in accordance with ATS guidelines. The best FEV1 and FVC values were selected from among those obtained in all acceptable maneuvers, whereas the FEF25-75%, FEF25%, and FEF75% values recorded were those obtained in the maneuvers with the highest sum of FVC and FEV1. Five children were not submitted to spirometry: 3 due to having recently used asthma medication or having recently had lower respiratory tract infections; and 2 because they declined to participate.
Subjects performed three to five maneuvers with a variation of less than 5% between the measurements and an expiratory time of at least 4 s. Calibration of the equipment was performed on a daily basis.
For the purposes of the present study, we considered primarily FEV1 and FVC, which are the most reproducible spirometric variables, as well as the ratio between the two. However, we also carried out an exploratory analysis using the instantaneous flows FEF25%, FEF75%, and FEF25-75%.
Skin prick tests were performed on the inner forearm, using standard dilutions of allergens in 50% glycerol. The allergens used were house dust mites, fungi, grass, and dust. Histamine was used as a positive control, and a negative control was also included.
The reactions were analyzed after 15 min and were considered positive when the induration diameter was ≥ 3 mm. The difference between allergen and negative control wheals should exceed 3 mm. Subjects were considered atopic if presenting at least one positive skin prick test.
Lung function variables were expressed as a percentage of predicted values, using the Hsu reference values,(12) and were defined as outcomes for the cross-sectional analyses performed at 6-7 years of age. Reference values adjusted for age, gender, and height were derived from the ATS criteria. Means and standard deviations were used in descriptive analysis. Linear regression was used to evaluate the association between outcomes and independent variables. The joint effect of the independent variables on the outcomes was analyzed by multiple linear regression using the robust option in the STATA statistical software (Stata Corp., College Station, TX, USA). Each lung function variable was analyzed in a separate model adjusted for all respiratory symptoms, as well as for social, biological, and environmental variables. All analyses were adjusted for gender and skin color. The significance level adopted for the exclusion or maintenance of any variable in the model was 0.05. Statistical analyses were weighted to compensate for oversampling of low birth weight subjects. The skewness of lung function variables was assessed, and transformations in FEV1, FVC, FEV1/FVC ratio, FEF25%, FEF50%, and FEF75% were not necessary. The FEF25-75% data were not normally distributed and showed great variability, with large standard deviations; these data were therefore excluded from analyses even after transformation.
Pearson's chi-square test and the chi-square test for linear trends were used to test the association between the exposure variables and wheezing patterns. The joint effect of independent variables on the outcomes was analyzed using Poisson regression. Given the binary nature and high prevalence of the outcome measure, we used the Poisson regression robust option (STATA Software), since the use of logistic regression might result in an overestimation of the prevalence rates.
The study design was approved by the Committee for Ethics in Research of the Federal University of Pelotas School of Medicine, as well as by the Brazilian Federal Medical Council. Written informed consent was obtained from mothers, and anonymity was guaranteed.
ResultsThe final sample included 494 children, representing 92.5% of the proposed sample. The main reason for loss to follow-up was change of address.
The majority of children were white (74.7%), female (52.8%), and from low-income families (59% of families earned ≤ 3× the minimum wage). More than 30% of children had been in contact with smoking before birth or at home during childhood (maternal smoking), one-quarter had a history of rhinitis apart from colds, and 46% presented positive skin tests for atopy.
Demographic characteristics, socioeconomic variables, and wheezing patterns are presented in Table 1. Of the 494 children who participated in this study, 290 (58.7%) had presented wheezing up to the age of 6 years.
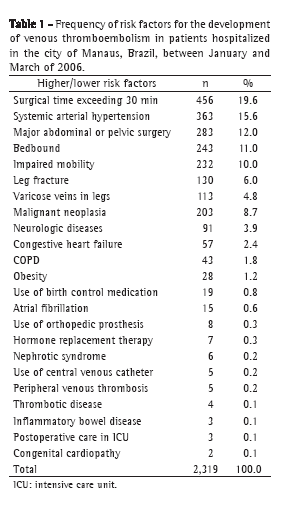
The prevalence of transient wheezing, persistent wheezing, and late-onset wheezing was 42.0%, 11.6%, and 5.3%, respectively. Rhinitis, a family history of asthma, and maternal smoking were more common among children with late-onset wheezing or persistent wheezing, and 43.9% of the latter group presented nonwhite skin color. Bronchiolitis in the first year of life was more common in the transient wheezing group.
The prevalence of asthma and respiratory symptoms is presented in Table 2, and lung function values are presented in Table 3. The prevalence of physician-diagnosed current asthma in 6-7 year olds was 12.8%. However, within the preceding 12 months, 16.8% of the children had presented wheezing, and 3.6% had experienced at least four wheezing episodes. Lung function values were within the normal range for all of the children studied. In order to identify any potential selection bias, the 143 children who were submitted to tests were compared to all other children in the cohort based on data collected at birth. The children tested did not differ significantly from the remainder of the cohort with respect to skin color, gender, family income, maternal education, preterm birth, low birth weight, or maternal smoking during pregnancy (data not shown).
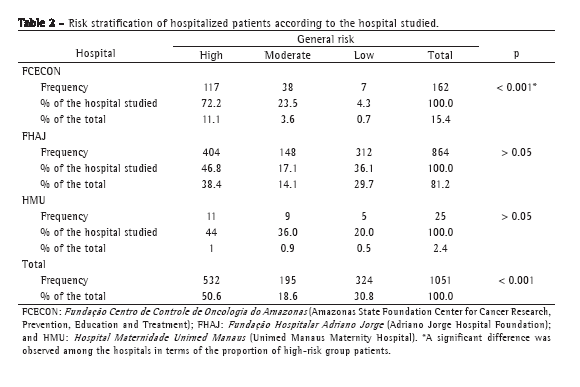

The distribution of lung function values according to social, biological, and environmental factors showed that nonwhite children presented FVC and FEV1 values that were lower than those observed for white children. No statistical differences were found in terms of gender, family income, preterm birth, low birth weight or maternal smoking.
Table 4 shows the results of the linear regression carried out for each lung function value and respiratory symptom. The statistical associations were adjusted for gender and skin color. Children with severe asthma-defined as having experienced at least four episodes of wheezing in the preceding 12 months, wheezing severe enough to limit speech, and sleep disturbance due to wheezing-presented lower lung function values, as did children with persistent wheezing. Mean FEV1/FVC ratio was lower in children with any of the following: current wheezing and asthma; asthma ever; at least four episodes of wheezing in the preceding 12 months; sleep disturbance due to wheezing; and exercise-induced wheezing.

Persistent wheezing was associated with lower FEV1/FVC ratio (β = −1.9, 95% CI: −3.6 to −0.2); mean FEV1/FVC ratio in children with transient wheezing was 4.9 points higher than in other children, and late-onset wheezing presented a positive association with FEV1, although the number of children in this category was small (Table 4).
Multiple linear regression was carried out for each lung function parameter and respiratory symptom in order to identify symptoms with independent effects on lung function. Models were adjusted for gender, skin color, and the other variables studied. Nonwhite skin color and wheezing severe enough to limit speech were independently associated with decreased mean FEV1. After adjusting for all asthma symptoms, as well as for social, environmental, and biological factors, we found the FEV1/FVC ratio to be positively associated with nonwhite skin color and negatively associated with exercise-induced wheezing.
Lower maternal level of education (mother having had less than eight years of schooling), bronchiolitis in first year of life, and a family history of asthma were independent risk factors for transient wheezing. Nonwhite skin color and family income > 10× the minimum wage were independently associated with persistent wheezing, the latter as a protective factor. At 6 years of age, children with persistent wheezing were more likely to have been officially diagnosed with asthma or bronchitis than were children with late-onset wheezing (71.9% vs. 42.3%).
DiscussionThis was the first study to investigate asthma and lung function in a birth cohort of Brazilian children. The high follow-up rate in this birth cohort ensures the representativeness of the studied sample, and the comparison of the sample as a whole with the subsample undergoing lung function tests and skin tests avoided any selection bias.
Of the 494 children who participated in this follow-up study, 290 (58.7%) had had episodes of wheezing before the age of 6 years. This proportion is higher than the 48.5% and 35.3% reported in two cohorts studies conducted in the United States and Germany, respectively.(12,14) The cohort analyzed in the present study was more comparable to the German cohort, since the prevalence of transient wheezing was above 70% in both studies. The prevalence of late-onset wheezing was lower in the present cohort than in the other cohorts studied.
Persistent wheezing was independently associated with nonwhite skin color and family income; children from families earning > 10× the minimum wage were protected against persistent wheezing. Children with persistent wheezing presented lower lung function at 6 years of age. Transient wheezing was associated with less maternal education (< 8 years of schooling), bronchiolitis in the first year of life, a family history of asthma, and normal lung function at age 6. Our lung function test results are in accordance with those reported in two previous studies,(12,14) although the association of persistent wheezing with family income and with skin color was not. Although a family history of asthma was associated with all wheezing patterns, in the multivariate analysis, it was found to be a risk factor for transient wheezing only.
In our sample, the prevalence of current asthma at the age of 6 years was 12.8%, and 16.8% of children showed symptoms of asthma at that age. At 4 years of age, the prevalence of asthma in our cohort was 18.4%, and 21.1% of children showed symptoms of asthma.(15) The decrease in the prevalence of asthma between the ages of 4 and 6 was not statistically significant. The prevalence of symptoms of severe asthma was lower in the present study than in a previous study.(7)
Many studies have shown that lung function in childhood can predict lung function in adult life.(16) Therefore, understanding the natural history of lung function and its possible risk factors is important for the prevention of pulmonary diseases.
Our results are in agreement with those of Delacourt et al.(17) and of the Tucson study,(18) in which persistent wheezers presented FEFs that were lower than those observed for transient wheezers. Based on those results, Delacourt et al. concluded that infantile asthma might be a risk factor for early loss of lung function, which is detected at 5 years of age and might be irreversible thereafter.
It is also known that some asthmatic children can present impaired lung function and therefore might be at high risk of developing lung diseases in the future. The reason for this is controversial: one hypothesis is that asthmatic children loose the elastic recoil of the lungs; another is that asthmatic children suffer growth disorders related to increased resting energy expenditure or inadequate energy intake.(19,20)
In the present study, children with respiratory symptoms of asthma presented impaired lung function. Since this was a longitudinal study, it allowed us to evaluate the different patterns of wheezing (transient, persistent and late-onset), as described elsewhere.(12) Although the prevalence of asthma and respiratory symptoms was high in our cohort, most of those children presented transient wheezing, rather than persistent or late-onset wheezing. The finding that lung function was impaired in 6-7 year olds with persistent wheezing who had not presented transient wheezing indicates that the persistence of wheezing can be a marker for risk in adulthood.
The FEV1/FVC ratio was affected by asthma, the children with asthma symptoms or having been diagnosed with asthma showing lower mean coefficients. Lower FEV1/FVC ratios were seen in children with persistent wheezing, indicating that some wheezing children already present impaired lung function at this early age.
Another interesting finding is that nonwhite children presented FEV1 values that were lower than those observed for white children, even after adjustment for socioeconomic factors. It should be noted that, at 4 years of age, the prevalence of asthma(15) was also higher among nonwhite children than among white children. It is well known that a great part of this difference is due to variations in the trunk to leg length ratio, blacks tending to have longer legs than do whites of the same height.(21)
In the present study, atopy, as assessed using skin tests, was not associated with impaired lung function, as was reported in a study conducted in Peru.(22) However, the prevalence of atopy in the present study was quite high. Although the association between asthma and atopy is not a consistent finding in the literature,(23) it could be argued that children with persistent asthma, rather than being atopic, have deficits in lung function.
Similarly, two other groups of researchers found that pulmonary function in asthmatic children was not influenced by atopy.(17,24) This suggests that factors other than atopy are involved in the onset of bronchial hyperreactivity in asthmatic children.
After adjustment for all other variables, exercise-induced wheezing was the most consistent independent risk factor for impaired lung function in our study, children with exercise-induced wheezing presenting a −4.4% mean reduction in the FEV1/FVC ratio. This was also seen in a study of 7-8 year olds in Belgium.(25)
Wheezing severe enough to limit speech, a marker of asthma severity, also remained significant after the multivariate analysis; children with such wheezing presented a −18.1% reduction in FEV1. This effect disappeared after adjustment for other lung function parameters, probably due to confounding factors.
Children with impaired lung function might be more susceptible to environmental factors and can be at an increased risk of declining lung function in adulthood. Therefore, persistent asthmatics should be identified and referred for treatment, so that lung function can be preserved and the prognosis improved. Further studies should be carried out in this population in order to analyze our finding that persistence of wheezing was strongly associated with family income and skin color.
References 1. Sly RM. Changing prevalence of allergic rhinitis and asthma. Ann Allergy Asthma Immunol. 1999;82(3):233-48; quiz 248-52.
2. Sears MR. Epidemiology of childhood asthma. Lancet. 1997;350(9083):1015-20.
3. Jenkins MA, Hopper JL, Bowes G, Carlin JB, Flander LB, Giles GG. Factors in childhood as predictors of asthma in adult life. BMJ. 1994;309(6947):90-3.
4. Werneck G, Ruiz S, Hart R, White M, Romieu I. Prevalence of asthma and other childhood allergies in Brazilian schoolchildren. J Asthma. 1999;36(8):677-90.
5. De Britto MC, Bezerra PG, Ferreira OS, Maranhao IC, Trigueiro GA. Asthma prevalence in schoolchildren in a city in north-east Brazil. Ann Trop Paediatr. 2000;20(2):95-100.
6. Camargos PA, Castro RM, Feldman JS. Prevalence of symptoms related to asthma in school children of Campos Gerais, Brazil [Article in Spanish]. Rev Panam Salud Publica. 1999;6(1):8-15.
7. Chatkin MN, Menezes AM, Albernaz E, Victora CG, Barros FC. Fatores de risco para consultas em pronto-socorro por criancas asmaticas no Sul do Brasil. Rev Saúde Pública. 2000;34(5):491-8.
8. Roorda RJ, Gerritsen J, Van Aalderen WM, Schouten JP, Veltman JC, et al. Risk factors for the persistence of respiratory symptoms in childhood asthma. Am Rev Respir Dis. 1993;148(6 Pt 1):1490-5.
9. Strachan D, Gerritsen J. Long-term outcome of early childhood wheezing: population data. Eur Respir J Suppl. 1996;21:S42-S47.
10. Oswald H, Phelan PD, Lanigan A, Hibbert M, Bowes G, Olinsky A. Outcome of childhood asthma in mid-adult life. BMJ. 1994;309(6947):95-6.
11. Solé D, Vanna AT, Yamada E, Rizzo MC, Naspitz CK. International Study of Asthma and Allergies in Childhood (ISAAC) written questionnaire: validation of the asthma component among Brazilian children. J Investig Allergol Clin Immunol. 1998;8(6):376-82.
12. Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332(3):133-8.
13. Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(3):1107-36.
14. Lau S, Illi S, Sommerfeld C, Niggemann B, Völkel K, Madloch C, et al. Transient early wheeze is not associated with impaired lung function in 7-yr-old children. Eur Respir J. 2003;21(5):834-41.
15. Chatkin MN, Menezes AM, Victora CG, Barros FC. High prevalence of asthma in preschool children in Southern Brazil: a population-based study. Pediatr Pulmonol. 2003;35(4):296-301.
16. Grol MH, Gerritsen J, Vonk JM, Schouten JP, Koëter GH, Rijcken B, et al. Risk factors for growth and decline of lung function in asthmatic individuals up to age 42 years. A 30-year follow-up study. Am J Respir Crit Care Med. 1999;160(6):1830-7.
17. Delacourt C, Benoist MR, Le Bourgeois M, Waernessyckle S, Rufin P, Brouard JJ, et al. Relationship between bronchial hyperresponsiveness and impaired lung function after infantile asthma. PLoS ONE. 2007;2(11):e1180.
18. Morgan WJ, Stern DA, Sherrill DL, Guerra S, Holberg CJ, Guilbert TW, et al. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am J Respir Crit Care Med. 2005;172(10):1253-8.
19. Hauspie R, Susanne C, Alexander F. Maturational delay and temporal growth retardation in asthmatic boys. J Allergy Clin Immunol. 1977;59(3):200-6.
20. Merkus PJ, van Essen-Zandvliet EE, Kouwenberg JM, Duiverman EJ, Van Houwelingen HC, Kerrebijn KF, et al. Large lungs after childhood asthma. A case-control study. Am Rev Respir Dis. 1993;148(6 Pt 1):1484-9.
21. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179-87.
22. Penny ME, Murad S, Madrid SS, Herrera TS, Piñeiro A, Caceres DE, et al. Respiratory symptoms, asthma, exercise test spirometry, and atopy in schoolchildren from a Lima shanty town. Thorax. 2001;56(8):607-12.
23. Pearce N, Pekkanen J, Beasley R. How much asthma is really attributable to atopy? Thorax. 1999;54(3):268-72.
24. Yang E, Kim W, Kwon BC, Choi SY, Sohn MH, Kim KE. Relationship among pulmonary function, bronchial hyperresponsiveness, and atopy in children with clinically stable asthma. Lung. 2006;184(2):73-9.
25. Droste JH, Wieringa MH, Weyler JJ, Nelen VJ, Van Bever HP, Vermeire PA. Lung function measures and their relationship to respiratory symptoms in 7- and 8-year-old children. Pediatr Pulmonol. 1999;27(4):260-6.
Trabalho realizado na Escola de Saúde da Universidade Católica de Pelotas - UCPel - e no Departamento de Clínica Médica da Universidade Federal de Pelotas - UFPel - Pelotas (RS) Brasil.
1. Diretora da Escola de Saúde da Universidade Católica de Pelotas - UCPel - Pelotas (RS) Brasil.
2. Professora Titular do Departamento de Clínica Médica da Universidade Federal de Pelotas - UFPel - Pelotas (RS) Brasil.
3. Professora do Departamento de Clínica Médica da Universidade Federal de Pelotas - UFPel - Pelotas (RS) Brasil.
4. Médico do Departamento de Clínica Médica da Universidade Federal de Pelotas - UFPel - Pelotas (RS) Brasil.
Endereço para correspondência: Moema Nudilemon Chatkin. Gonçalves Chaves, 3625/304, CEP 96015-560, Pelotas, RS, Brasil.
Tel 53 2128-8501. E-mail: moemachatkin@gmail.com
Apoio financeiro: Nenhum.
Recebido para publicação em 3/6/2007. Aprovado, após revisão, em 11/2/2008.
**A versão completa em português deste artigo está disponível em www.jornaldepneumologia.com.br







