ABSTRACT
Objective: To evaluate the short-term impact of tiotropium in patients with severe or very severe COPD who complain of dyspnea despite being currently treated with other bronchodilators. Methods: A prospective study including patients with severe or very severe COPD and complaining of dyspnea at rest or on minimal exertion. Every 15 days, the bronchodilator treatment regimen was altered, from salmeterol to tiotropium to salmeterol+tiotropium. At the end of each regimen, pulmonary function tests and the six-minute walk test (6MWT) were performed. The degree of dyspnea and the ability to perform activities of daily living were also assessed. To evaluate patient ability to perform activities of daily living, we employed the London Chest Activity of Daily Living (LCADL), validated for use in Brazil. Results: We evaluated 52 patients, 30 of whom completed the study. The use of tiotropium in isolation resulted in significant improvement in dyspnea at baseline (mean Medical Research Council scale score reduced from 3.0 to 2.5) and at the end of 6MWT (mean Borg scale score reduced from 6.1 to 4.5), and the differences were significant (p < 0.05 for both). The use of the salmeterol+tiotropium combination resulted in a significant (81 mL) increase in FEV1 and a 5.7 point improvement in the LCADL score. Conclusions: The introduction of tiotropium into the treatment of patients with severe or very severe COPD and using long-acting β2 agonists improves pulmonary function and provides symptomatic relief, as perceived by patients in the short term. These results, obtained under real life treatment conditions, support the use of the salmeterol+tiotropium combination in specific treatment protocols for these patients.
Keywords:
Pulmonary disease, chronic obstructive; Bronchodilator agents; Dyspnea; Activities of daily living.
RESUMO
Objetivo: Avaliar o impacto de curto prazo do uso de tiotrópio em pacientes com DPOC grave e muito grave com queixas de dispneia apesar do tratamento com outros broncodilatadores. Métodos: Estudo prospectivo incluindo pacientes com DPOC grave ou muito grave, com queixa de dispneia de pequenos esforços ou ao repouso. A cada 15 dias, o tratamento broncodilatador foi modificado: salmeterol, tiotrópio e associação salmeterol+tiotrópio. Ao final de cada regime, foram realizados testes de função pulmonar e teste de caminhada de seis minutos (TC6). Também foram avaliados o grau de dispneia e a capacidade de realização de atividades de vida diária. Para a avaliação das atividades de vida diária, foi utilizada a escala London Chest Activity of Daily Living (LCADL) validado para uso no Brasil. Resultados: Foram avaliados 52 pacientes. Desses, 30 completaram o estudo. A introdução de tiotrópio como monoterapia resultou em uma melhora significativa (p < 0,05) da dispneia basal (média do escore da escala do Medical Research Council de 3,0 para 2,5) e ao final do TC6 (média do escore da escala de Borg de 6,1 para 4,5), e as diferenças foram significativas (p < 0,05 para ambos). O uso da associação salmeterol+tiotrópio resultou em um aumento significativo médio de 81 mL no VEF1 e na melhora de 5,7 pontos no escore da escala LCADL. Conclusões: A introdução de tiotrópio no tratamento de pacientes com DPOC grave a muito grave em uso de β2-agonistas de longa duração causa melhora na função pulmonar e alivio sintomático perceptível pelos pacientes a curto prazo. Esses resultados, obtidos em regime de atendimento de vida real, dão suporte ao uso da associação salmeterol+tiotrópio em protocolos de assistência específicos a esses pacientes.
Palavras-chave:
Doença pulmonar obstrutiva crônica; Broncodilatadores; Dispneia; Atividades cotidianas.
IntroductionThe respiratory disease known as COPD is characterized by airflow obstruction that is not fully reversible and is associated with an abnormal inflammatory response to the inhalation of cigarette smoke, noxious particles or toxic gases. This disease results in progressive airflow limitation and systemic manifestations, such as an increased risk for cardiovascular diseases, hypoxemia, depression and physical deconditioning.(1)
The injury to the alveolar support structure, the increased airway resistance and the excess secretion lead to chronic airflow limitation, which causes progressive dyspnea and intolerance to physical activity. Patients then begin to reduce their physical activities, and this leads to a downward spiral of deconditioning and exercise intolerance, which is clear in the anamnesis and can be confirmed by exercise tests.(2)
Since COPD is a chronic progressive disease that is only minimally reversible, the objective of treatment is to control the symptoms and reduce disease progression. With the reduction in dyspnea and the increase in exercise tolerance, it is possible to offer a better quality of life to patients.(3)
Long-acting β2 agonists (salmeterol and formoterol) and long-acting anticholinergics (tiotropium) are the bronchodilators of choice for the maintenance treatment of patients with dyspnea that is not controlled with short-acting drugs. Various authors have endorsed the use of such treatment as monotherapy.(4-8)
Even before there was evidence in the literature, the combination of bronchodilators with different mechanisms of action was advocated for improving the efficacy of treatment and reducing the risk of side effects in relation to the increase in the dose of a single bronchodilator.(3) The current recommendation is that symptomatic patients diagnosed with at least moderate COPD, as defined by the Global Initiative for Chronic Obstructive Lung Disease (GOLD)-FEV1/FVC ratio < 0.7 and FEV1 < 80% of predicted-should receive treatment with one or more long-acting bronchodilators. Whether to use these drugs to control COPD, as well as which bronchodilator should be used, is decided on the basis of the symptoms presented and on the patient response to each drug.(1,9)
In view of the physiological and clinical benefits of tiotropium and the efficacy of long-acting β2 agonists, it is intuitive to assume that combined long-acting bronchodilator therapy would result in additional benefits. In fact, combined long-acting bronchodilator treatment seems to add long-term benefits to pulmonary function and quality of life, without any apparent harm or any significant side effects.(10)
The short-term clinical benefits of the use of tiotropium in isolation or in combination with a long-acting β2 agonist have been little studied.(11)
Rapid improvement of symptoms not only benefits patients, by ridding them of an inconvenience, but also increases adherence to and confidence in the current therapeutic regimen.
The objective of the present study was to evaluate the short-term impact of
the use of tiotropium in isolation or in combination with a long-acting β2 agonist in patients with severe or very severe COPD who complain of dyspnea despite being currently treated with other bronchodilators.
MethodsThis was an open, nonrandomized, prospective study including patients diagnosed with COPD (GOLD stages III and IV) who were monitored at our facility. The patients were selected during outpatient treatment. The physician responsible for the outpatient treatment referred eligible patients to the protocol team. The inclusion criteria were as follows: having had no changes in medication and no exacerbations in the last 30 days; being under regular treatment with a long-acting β2 agonist (salmeterol or formoterol); having an FEV1 < 50% of predicted after bronchodilator use; and scoring between 3 and 4 on the Medical Research Council (MRC) dyspnea questionnaire.(12)
Patients who had an orthopedic or cognitive impairment that prevented them from performing the six-minute walk test (6MWT) or from completing the questionnaires were excluded from the study. Those with a history of tiotropium use were also excluded. Other bronchodilators were allowed.
At the selection visit, on day zero of the study (D0), patients were assessed in terms of the inclusion and exclusion criteria by a single researcher.
Subsequently, patients underwent simple spirometry, in which the procedures followed were those recommended in the guidelines established by the Brazilian Thoracic Association (BTA),(13) and completed the MRC questionnaire in order to determine the degree of dyspnea, confirming patient eligibility for the protocol. All participating patients gave written informed consent. At the end of the visit, each patient received a prescription for salmeterol (50 µg every 12 h) and taught how to use the device. Since all the patients selected should be regularly using a long-acting inhaled β2 agonist (formoterol or salmeterol), the first 15 days were a run-in period whose only aim was to standardize the bronchodilator treatment.
The patients who met the inclusion criteria were monitored at three subsequent visits conducted every 15 days. The degree of dyspnea of the COPD patients, as well as their exercise capacity, their ability to perform activities of daily living and their pulmonary function, was assessed in sequential two-week periods as the bronchodilator treatment regimen was altered.
The degree of dyspnea was assessed by the MRC scale. Exercise capacity was assessed by the 6MWT, in accordance with the American Thoracic Society recommendations.(14) At the end of the 6MWT, the following parameters were determined: six-minute walk distance (6MWD); HR; RR; SpO2; and the degree of dyspnea (Borg scale). To evaluate patient ability to perform activities of daily living, we employed the London Chest Activity of Daily Living (LCADL) scale, validated for use in Brazil.(15-
17)
At the second visit (D15), the spirometric measurements were taken again and the degree of dyspnea was reassessed. Patients who scored between 3 and 4 on the MRC dyspnea questionnaire and had a post-bronchodilator FEV1 < 50% of predicted were maintained in the protocol, since the objective of the study was to evaluate patients who continued to experience dyspnea on minimal exertion despite the use of a long-acting β2 agonist. Those who showed improvement in functional class or a decrease in the degree of dyspnea between D0 and D15 were excluded from the study, as were those who had an exacerbation or who did not adhere to the prescribed treatment in those first two weeks.
At D15, the LCADL scale was administered and the 6MWT was performed.
At the end of D15, the use of salmeterol was discontinued and a 15-day course of tiotropium (18 µg once a day) was prescribed. At that point, the patients were instructed in the proper technique for inhalation with the use of a capsule device.
On D30, all the measurements were taken again and the questionnaires were re-administered. At the end of that day, the combination of salmeterol and tiotropium bromide was prescribed. At the fourth, and final, evaluation (on D45), the same parameters were again assessed.
Throughout the protocol, the other drugs used for the treatment of COPD and of other associated diseases were maintained. However, the use of any other anticholinergics was not allowed.
The pulmonary function test results and the MRC scale scores obtained at the four visits were compared by ANOVA and the Holm-Sidak method for multiple comparisons. The LCADL scores and the 6MWT results obtained at D15, D30 and D45 were compared by the same statistical program (Sigma Plot v.10.0; SPSS Inc., Chicago, IL, USA).Values of p < 0.05 were considered statistically significant.
The research project was approved by the Research Ethics Committee of the University of São Paulo School of Medicine Hospital das Clínicas.
ResultsThe population evaluated included 52 patients. Of those, 5 were excluded: 2 due to orthopedic impairment that prevented them from performing the 6MWT; and 3 due to inability to understand the questionnaires. In addition, another 17 did not complete the study: 5 due to exacerbations between D0 and D15; and 12 due to lack of adherence to the treatment prescribed, observed at D15. None of the selected patients were excluded due to improvement in functional class or to a decrease in the degree of dyspnea between D0 and D15.
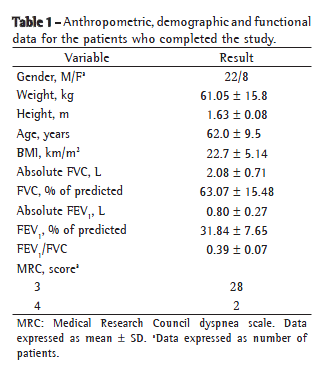
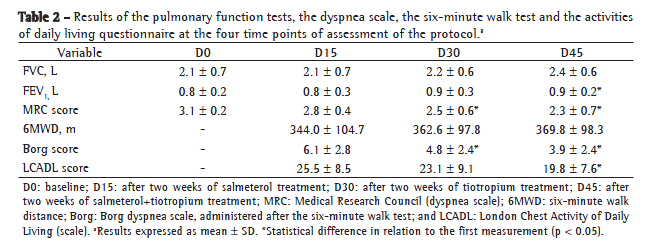
Table 1 shows the demographic, anthropometric and functional data related to the 30 patients who completed the study. Table 2 summarizes the results of the protocol at the four visits.
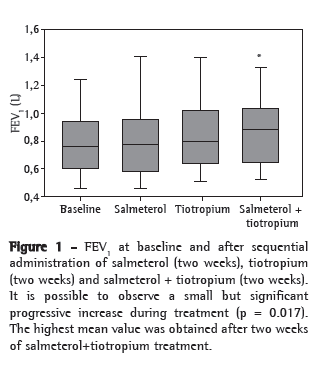
Spirometry showed that, although there was a mean increase of 165 mL in FVC between D0 and D45, this variation was not statistically significant (p = 0.68). There was a significant increase in FEV1 during treatment (mean increase of 81 mL between D0 and D45; p = 0.017). The increase in pulmonary function was progressive during the protocol period (Figure 1).
Dyspnea, as measured by the MRC scale, presented a gradual reduction at all visits. There was no statistically significant difference between the mean MRC score obtained at D0 and that obtained at D15 (3.06 vs. 2.83).
The mean MRC score dropped to 2.50 at D30 (after the use of tiotropium) and to 2.26 at D45 (after the use of the salmeterol-tiotropium combination), both of which were statistically significant reductions in relation to the baseline measurement (p < 0.05 for both).
At D15, the mean 6MWD was 343 m. At D30 and D45, respectively, the mean 6MWD was 362 m and 369 m. There were no statistically or clinically significant differences between the three visits in terms of the 6MWD values obtained. The baseline and final values for HR, RR and SpO2 obtained were similar for all three 6MWTs.
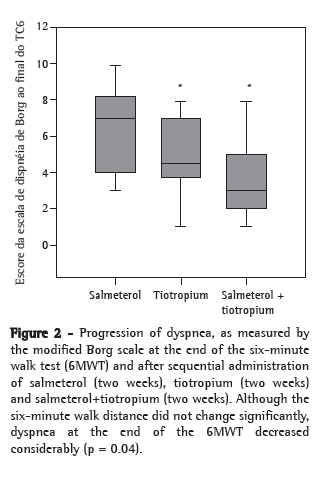
When assessing the degree of dyspnea, as measured by the Borg scale at the end of the 6MWT, however, we observed a considerable reduction, from a mean value of 6.1 at D15 to 4.8 at D30 and to 3.8 at D45 (p = 0.004; Figure 2).
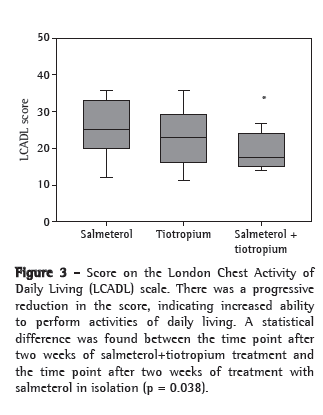
Patient ability to perform activities of daily living was assessed by the LCADL scale at the last three visits. The mean values were 25.4, 23.1 and 19.7, respectively, at D15, D30 and D45. This progressive reduction during the protocol period shows significantly decreased limitation with the use of the salmeterol-tiotropium combination (p = 0.038; Figure 3).
None of our patients had exacerbations or reported any significant side effects during the period between the introduction of the salmeterol-tiotropium combination and the end of the 45-day follow-up period.
DiscussionThe combined administration of a β2 agonist and a long-acting anticholinergic to patients with severe or very severe COPD resulted in short-term symptomatic and functional improvement, which translated to an increase in their ability to perform activities of daily living. This response was superior to that obtained with the use of each drug in isolation. These results support the use of drugs in combination in COPD patients who remain symptomatic with the use of drugs in isolation.
The design of our study, which was carried out under real life conditions at a university hospital, followed the official medication distribution protocol defined by the São Paulo State Department of Health.(18) Considering the proven efficacy of β2 agonists and long-acting anticholinergics,(4,10,19)
that protocol defines the first option as the β2 agonist, due to its lower cost.
Therefore, at the time of inclusion, our patients were currently using a β2
agonist, albeit with little response to the treatment.
In the first two weeks (the protocol run-in period), we maintained the treatment with the β2 agonist, making sure that the patients used the device appropriately and adhered to the treatment prescribed. We therefore ensured that the comparison among the three periods of the protocol was performed under similar conditions.
Although the patients reported a reduction in dyspnea after two weeks of tiotropium treatment in comparison with the period of treatment with salmeterol in isolation, this difference should be interpreted with caution.
In accordance with the protocol, those who were found to be clinically compensated with long-acting β2 agonists were not included. Therefore, we cannot conclude that one bronchodilator was more effective than the other.
The benefits of tiotropium have been proven in several studies. In 2002, a one-year randomized, double-blind, placebo-controlled study was conducted with the objective of evaluating the long-term use of a daily dose of tiotropium. The result was that the use of tiotropium led to a sustained improvement in pulmonary function, a reduction in dyspnea, a better performance on quality-of-life questionnaires and fewer exacerbations in comparison with the use of a placebo.(6)
In 2004, a randomized, double-blind, placebo-controlled study evaluated the effects of tiotropium on lung hyperinflation, dyspnea and exercise tolerance. After 42 days of use, there was a reduction in lung hyperinflation at rest and on exertion. The change in respiratory mechanics allowed an increase in exercise capacity and a reduction in dyspnea.(7)
Few studies have compared the efficacy of β2 agonists with that of tiotropium. One group of authors, evaluating the acute 24-h response to those drugs in two pilot studies, demonstrated that formoterol and salmeterol have a more rapid onset of action than does tiotropium and produce maximal bronchodilation superior to that produced by tiotropium; conversely, tiotropium is longer acting.(20,21)
Salmeterol and tiotropium have been compared in longer studies.(22,23) In general, those studies suggest that the bronchodilator and clinical response produced by tiotropium are slightly superior but of little clinical significance. As a consequence, recent guidelines suggest that symptomatic patients with COPD should be initially treated with a long-acting bronchodilator in isolation and that there is insufficient evidence to recommend one drug over the other.(24) Our findings allow us to infer that patients who continue to have class III to IV dyspnea, as measured by the MRC scale, despite the use of β2 agonists, can benefit from the use of tiotropium in isolation. More recently, the combination of these two drugs has been evaluated. In a one-year study of 449 patients, it was demonstrated that adding tiotropium to the salmeterol-fluticasone combination had a beneficial effect on quality of life, pulmonary function and hospital admission rates, when compared with the use of tiotropium in isolation.(25)
In a study of 71 patients, the use of the tiotropium-formoterol combination resulted in more marked improvement in pulmonary function after a period of six weeks than did the use of each of the two drugs in isolation. The authors found no increase in the rates of side effects among the patients receiving the combined therapy.(26)
In our population of patients (with severe or very severe COPD and symptomatic despite the use of salmeterol or tiotropium), the two-drug combination resulted in improvement, as perceived by patients in the short term. The statistically significant increase in FEV1 and the improvement in the dyspnea scores affected the daily routine of patients, as evidenced by the better score on the activities of daily living questionnaire.
Few studies have evaluated the short-term efficacy of the combination of β2 agonists and tiotropium. In both of the studies previously mentioned,(20,21) the spirometric response to the drug combination was superior to that obtained with the use of each drug in isolation.
One study was similar to ours in that it compared the use of tiotropium in isolation or combined with a β2 agonist for a period of two weeks. That study included 95 patients with moderate to severe COPD who received tiotropium for two weeks as baseline treatment, followed by three periods of two weeks each, in which they were treated with tiotropium in isolation, tiotropium combined with formoterol once a day and tiotropium combined with formoterol twice a day. The drug combination, with a once or twice daily dose of formoterol, resulted in a significant increase in FEV1. Clinical outcomes were not analyzed.(27)
In a short-term (one-week) study, 20 patients with moderate to severe COPD were treated for a week by means of a cross design of three regimens: salmeterol+fluticasone twice a day; tiotropium once a day + fluticasone twice a day; and the combined treatment with salmeterol+fluticasone twice a day and tiotropium once a day. There were no significant differences between the use of tiotropium and the use of salmeterol in terms of the subsequent spirometry findings. However, FEV1 was significantly higher after the combined treatment. Clinical outcomes were also not examined in that study.(28)
In our study, we evaluated only 30 of the 52 patients initially selected. A considerable proportion of patients did not complete the study because they had an exacerbation during the selection process or because they were later found to be non-adherent.
In our study, a high proportion of the patients (12 of 52) had poor adherence. Our sample consisted of patients who continued to have limiting symptoms despite being currently treated with a bronchodilator. Since low adherence to the therapeutic regimen is one of the reasons for the difficulty in controlling dyspnea, it is plausible that the number of non-adherent patients was above the mean in this particular group studied.
Due to the small sample size and the considerable number of patients excluded, the power of the study is low, which can lead to a type II error (not finding an effect where one exists). However, even with low statistical power, it was possible to observe clinical and functional improvement.
In addition, since patients who adhered poorly to the prescribed treatment were excluded, the external validity of this study is likely to apply only to patients who are motivated and adhere to the drug treatment offered.
Another limitation of the study is the lack of a control group. Therefore, it is possible that the result observed was due to the duration of treatment or to the placebo effect rather than to the change in the prescribed drug.
However, we emphasize that, at the time of their inclusion in the study, all of our patients were currently being treated with a long-acting β2 agonist in isolation, had not achieved satisfactory symptom control and continued to experience dyspnea on minimal exertion. This fact minimizes the chance of an effect attributed only to the duration of treatment.
It is also noteworthy that, when analyzing the outcomes of the study, we demonstrated that the use of salmeterol+tiotropium in symptomatic patients previously being treated with one drug in isolation led to such improvement in functional and clinical parameters that their performance of activities of daily living improved. The gains were both clinical and functional, which is in agreement with the findings of the studies previously cited, and did not seem to result from the placebo effect. The two drugs seem to have a true additive effect, even in the short term.
Long-acting β2 agonists bind to receptors of the smooth muscles of the bronchi and increase the G protein stimulus. This leads to muscle fiber relaxation and bronchodilation. Therefore, the effect is increased if the fiber is contracted.(29) Pharmacokinetically, tiotropium presents selectivity for the muscarinic receptors M1 and M3. Due to its affinity for M3 receptors, tiotropium is long acting and is used in a single daily dose. It has few side effects and is quite safe.(30)
The introduction of tiotropium into the treatment of COPD not only increases bronchodilation but also changes the way in which the effect is obtained. Cholinergic bronchomotor tone is of little relevance in normal individuals. In patients with COPD, however, its reduction changes the radius of the airways in their state of relaxation, having a significant effect, since small variations in the caliber of the airways alter resistance to the power of 4.
Guidelines for COPD (GOLD and the BTA Consensus) recommend the administration of long-acting bronchodilators to symptomatic patients with COPD being treated with short-acting rescue medication. These documents emphasize that the treatment should be adapted to the local conditions, including the resources available and the individual patient characteristics.
The treatment protocol for COPD in the state of São Paulo(18)has been developed based on these concepts. Considering the debatable clinical difference between long-acting β2 agonists and tiotropium in terms of effect, the protocol designates the use of a β2 agonist in isolation as the treatment of choice.Our study, designed to evaluate the practical applicability of the protocol, supports the assertion that the combination of tiotropium and β2 agonists is beneficial in patients with severe or very severe COPD.
In summary, we demonstrated that the introduction of tiotropium into the treatment regimen of patients with severe or very severe COPD improves pulmonary function and provides symptomatic relief, as perceived by patients in the short term. These results, obtained under real life treatment conditions, support the use of the salmeterol-tiotropium combination in specific treatment protocols for these patients.
Acknowledgments
The authors would like to thank Aline Lickel Pimentel for her help in formatting the illustrations.
References
1. Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532-55.
2. Gallagher CG. Exercise limitation and clinical exercise testing in chronic obstructive pulmonary disease. Clin Chest Med. 1994;15(2):305-26.
3. Diaz PT, Bruns AS, Ezzie ME, Marchetti N, Thomashow BM. Optimizing bronchodilator therapy in emphysema. Proc Am Thorac Soc. 2008;5(4):501-5.
4. Mahler DA, Donohue JF, Barbee RA, Goldman MD, Gross NJ, Wisniewski ME, et al. Efficacy of salmeterol xinafoate in the treatment of COPD. Chest. 1999;115(4):957-65.
5. Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543-54.
6. Vincken W, van Noord JA, Greefhorst AP, Bantje TA, Kesten S, Korducki L, et al. Improved health outcomes in patients with COPD during 1 yr's treatment with tiotropium. Eur Respir J. 2002;19(2):209-16.
7. O'Donnell DE, Flüge T, Gerken F, Hamilton A, Webb K, Aguilaniu B, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J. 2004;23(6):832-40.
8. Rubin AS, Souza FJ, Hetzel JL, Moreira Jda S. Immediate bronchodilator response to formoterol in poorly reversible chronic obstructive pulmonary disease. J Bras Pneumol. 2008;34(6):373-9.
9. Sociedade Brasileira de Pneumologia e Tisiologia. II Consenso Brasileiro sobre Doença Pulmonar Obstrutiva Crônica. J Pneumol. 2004;30(Suppl 5):S1-S42.
10. Tashkin DP, Cooper CB. The role of long-acting bronchodilators in the management of stable COPD. Chest. 2004;125(1):249-59.
11. Reddy CB, Kanner RE. Is combination therapy with inhaled anticholinergics and beta2-adrenoceptor agonists justified for chronic obstructive pulmonary disease? Drugs Aging. 2007;24(8):615-28.
12. Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580-6.
13. Sociedade Brasileira de Pneumologia e Tisiologia. Diretrizes para Testes de Função Pulmonar. J Pneumol. 2002;28(Suppl 3):S1-S238.
14. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111-7.
15. Garrod R, Paul EA, Wedzicha JA. An evaluation of the reliability and sensitivity of the London Chest Activity of Daily Living Scale (LCADL). Respir Med. 2002;96(9):725-30.
16. Garrod R, Bestall JC, Paul EA, Wedzicha JA, Jones PW. Development and validation of a standardized measure of activity of daily living in patients with severe COPD: the London Chest Activity of Daily Living scale (LCADL). Respir Med. 2000;94(6):589-96.
17. Carpes MF, Mayer AF, Simon KM, Jardim JR, Garrod R. The Brazilian Portuguese version of the London Chest Activity of Daily Living scale for use in patients with chronic obstructive pulmonary disease. J Bras Pneumol. 2008;34(3):143-51.
18. Dispensação de medicamentos a portadores de DPOC. Diário Oficial do Estado São Paulo, SP. 2007;Seção 1(10 ago/2007):44.
19. Jones PW, Bosh TK. Quality of life changes in COPD patients treated with salmeterol. Am J Respir Crit Care Med. 1997;155(4):1283-9.
20. Cazzola M, Centanni S, Santus P, Verga M, Mondoni M, di Marco F, et al. The functional impact of adding salmeterol and tiotropium in patients with stable COPD. Respir Med. 2004;98(12):1214-21.
21. Cazzola M, Di Marco F, Santus P, Boveri B, Verga M, Matera MG, et al. The pharmacodynamic effects of single inhaled doses of formoterol, tiotropium and their combination in patients with COPD. Pulm Pharmacol Ther. 2004;17(1):35-9.
22. Brusasco V, Hodder R, Miravitlles M, Korducki L, Towse L, Kesten S. Health outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPD. Thorax. 2003;58(5):399-404. Erratum in: Thorax. 2005;60(2):105.
23. Donohue JF, van Noord JA, Bateman ED, Langley SJ, Lee A, Witek TJ Jr, et al. A 6-month, placebo-controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterol. Chest. 2002;122(1):47-55.
24. Qaseem A, Snow V, Shekelle P, Sherif K, Wilt TJ, Weinberger S, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2007;147(9):633-8.
25. Aaron SD, Vandemheen KL, Fergusson D, Maltais F, Bourbeau J, Goldstein R, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2007;146(8):545-55.
26. van Noord JA, Aumann JL, Janssens E, Smeets JJ, Verhaert J, Disse B, et al. Comparison of tiotropium once daily, formoterol twice daily and both combined once daily in patients with COPD. Eur Respir J. 2005;26(2):214-22.
27. van Noord JA, Aumann JL, Janssens E, Verhaert J, Smeets JJ, Mueller A, et al. Effects of tiotropium with and without formoterol on airflow obstruction and resting hyperinflation in patients with COPD. Chest. 2006;129(3):509-17.
28. Baloira Villar A, Vilariño Pombo C. Bronchodilator efficacy of combined salmeterol and tiotropium in patients with chronic obstructive pulmonary disease [Article in Spanish]. Arch Bronconeumol. 2005;41(3):130-4.
29. Nelson HS. Beta-adrenergic bronchodilators. N Engl J Med. 1995;333(8):499-506.
30. Barnes PJ. The pharmacological properties of tiotropium. Chest. 2000;117(2 Suppl):63S-6S.
* Study carried out at the Division of Pulmonology of the Instituto do Coração, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo - InCor/HC-FMUSP, Heart Institute/University of São Paulo School of Medicine Hospital das Clínicas - São Paulo, Brazil.
Correspondence to: Frederico Leon Arrabal Fernandes. Sec. Pneumologia, Avenida Dr. Enéas de Carvalho Aguiar, 44, CEP 05403-900, São Paulo, SP, Brasil.
Tel 55 11 3069-5034. Email: pnefred@incor.usp.br
Financial support: None.
Submitted: 6 July 2009. Accepted, after review: 26 November 2009.
About the authorsFrederico Leon Arrabal Fernandes
Attending Physician. Department of Pulmonology, Instituto do Coração, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo - InCor/HC-FMUSP, Heart Institute/University of São Paulo School of Medicine Hospital das Clínicas - São Paulo, Brazil.
Vanessa Aparecida Leão Pavezi
Physical Therapist. Pulmonary Function Laboratory, Instituto do Coração, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo - InCor/HC-FMUSP, Heart Institute/University of São Paulo School of Medicine Hospital das Clínicas - São Paulo, Brazil.
Sérvulo Azevedo Dias Jr
Graduate Student. Department of Pulmonology, Instituto do Coração, Hospital das Clínicas da Faculdade de Medicina da Universidade de São
Paulo - InCor/HC-FMUSP, Heart Institute/University of São Paulo School of Medicine Hospital das Clínicas - São Paulo, Brazil.
Regina Maria Carvalho Pinto
Attending Physician. Department of Pulmonology, Instituto do Coração, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo - InCor/HC-FMUSP, Heart Institute/University of São Paulo School of Medicine Hospital das Clínicas - São Paulo, Brazil.
Rafael Stelmach
Attending Physician. Department of Pulmonology, Instituto do Coração, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo - InCor/HC-FMUSP, Heart Institute/University of São Paulo School of Medicine Hospital das Clínicas - São Paulo, Brazil.
Alberto Cukier
Full Professor. Department of Pulmonology, Faculdade de Medicina da Universidade de São Paulo - FMUSP, University of São Paulo School of Medicine - São Paulo, Brazil.








