ABSTRACT
Objective: To determine whether the variation in lung function over one year is associated with worse clinical outcomes, as well as with a decline in lung function in the following years, in patients with cystic fibrosis (CF). Methods: This was a retrospective study involving CF patients (4-19 years of age), evaluated over a three-year period. We evaluated demographic characteristics, chronic Pseudomonas aeruginosa infection, antibiotic use, hospitalization, six-minute walk distance (6MWD), and lung function. The inclusion criterion was having undergone pulmonary function testing at least three times in the first year and at least once in each of the next two years. Results: We evaluated 35 CF patients. The variation in FEV1 in the first year (ΔFEV1) was greater among those who, in the third year, showed reduced FEV1, had a below-average 6MWD, or were hospitalized than among those with normal FEV1, normal 6MWD, or no hospital admissions, in that same year (p < 0.05), although no such difference was found for antibiotic use in the third year. Subjects showing a ΔFEV1 ≥ 10% also showed a greater decline in FEV1 over the two subsequent years (p = 0.04). The ΔFEV1 also showed an inverse correlation with absolute FEV1 in the third year (r = −0.340, p = 0.04) and with the rate of FEV1 decline (r = −0.52, p = 0.001). Linear regression identified ΔFEV1 as a predictor of FEV1 decline (coefficient of determination, 0.27). Conclusions: Significant variation in lung function over one year seems to be associated with a higher subsequent rate of FEV1 decline and worse clinical outcomes in CF patients. Short-term ΔFEV1 might prove useful as a predictor of CF progression in clinical practice.
Keywords:
Cystic fibrosis; Respiratory function tests; Disease progression; Hospitalization; Forced expiratory volume.
RESUMO
Objetivo: Determinar se a variação na função pulmonar em um ano está associada com piores desfechos clínicos e declínio da função pulmonar nos anos seguintes em pacientes com fibrose cística (FC). Métodos: Estudo retrospectivo incluindo pacientes com FC (4-19 anos de idade), avaliados por um período de três anos. Avaliamos características demográficas, infecção crônica por Pseudomonas aeruginosa, uso de antibióticos, internação hospitalar, distância percorrida no teste de caminhada de seis minutos (DTC6) e função pulmonar. Os critérios de inclusão foram ter sido submetido a testes de função pulmonar por ao menos três vezes no primeiro ano e a pelo menos um teste em cada um dos dois anos subsequentes. Resultados: Foram avaliados 35 pacientes com FC. A variação do VEF1 no primeiro ano (ΔVEF1) foi maior entre aqueles que, no terceiro ano, apresentaram VEF1 reduzido, DTC6 abaixo do normal ou que foram hospitalizados do que entre aqueles que apresentaram VEF1 normal, DTC6 normal ou sem hospitalização naquele mesmo ano (p < 0,05), embora não tenha havido tal diferença em relação ao uso de antibióticos no terceiro ano. Os pacientes com ΔVEF1 ≥ 10% também apresentaram maior declínio do VEF1 ao longo dos dois anos subsequentes (p = 0,04). A ΔVEF1 também apresentou uma correlação inversa com o VEF1 no terceiro ano (r = −0,340; p = 0,04) e com a taxa de declínio do VEF1 (r = −0,52; p = 0,001). A regressão linear identificou ΔVEF1 como um preditor da taxa de declínio do VEF1 (coeficiente de determinação = 0,27). Conclusões: Variações significativas na função pulmonar em um ano parecem estar associadas com uma maior taxa de declínio do VEF1 e piores desfechos clínicos nos anos subsequentes em pacientes com FC. A ΔVEF1 de curto prazo pode ser útil como um preditor da progressão da FC na prática clínica.
Palavras-chave:
Fibrose cística; Testes de função respiratória; Progressão da doença; Hospitalização; Volume expiratório forcado no primeiro segundo.
INTRODUCTIONCystic fibrosis (CF) is a genetic disease, with a chronic evolution, that compromises the normal function of various organs and systems. It is characterized by changes in the secretions of the respiratory and gastrointestinal tract.(1) It is most common (at 1/3,500 live births) in the White population.(2,3) It is a progressive condition in which lung disease is the major determinant of morbidity and mortality.(4)
Due to advances in the treatment and understanding of CF, there has been a significant increase in life expectancy of individuals suffering from the disease. In Europe, CF survival has reached a mean age of approximately 35 years.(3,5) Estimates showed that subjects born after 2000 will have a life expectancy of over 50 years of age.(2,5) However, the progressive decline in lung function over time appears to be an inevitable characteristic of the disease in nearly all cases.(4) Therefore, impaired lung function, as quantified by measuring FEV1, expressed as a percentage of the predicted value, is one of the main markers affecting clinical decision making about changing or intensifying the treatment regimens employed in CF patients.(6,7)
In recent decades, the FEV1 of subjects with CF has been studied in order to gain a better understanding of the pro-gression of the associated lung disease and to identify risk groups in which more aggressive therapy is indicated.(7-9) A decline in FEV1 has been reported to be a marker of greater risk of hospitalization and death in subjects with chron-ic obstructive pulmonary diseases,(6,10) as well as being considered the best single indication for lung transplantation. Previous findings have shown that 80% of CF-related deaths are directly or indirectly associated with reduced lung function.(11)
The risk factors most commonly associated with a progressive decline in FEV1 among CF patients include advanced age, female gender, a F508 mutation in the CF transmembrane conductance regulator, the presence of modifier genes, pancreatic insufficiency, low nutritional status, diabetes mellitus, and colonization of the respiratory tract by Pseudomonas aeruginosa or Burkholderia cepacia. In addition, daily production of sputum, wheezing, and the num-ber of lung exacerbations treated with intravenous antibiotics also seem to be related to a decline in lung function among CF patients.(7,12,13) The significance and magnitude of the effects of these factors seem to depend on patient age.(13) Furthermore, recent findings suggest that a reduction in the six-minute walk distance (6MWD) is associated with greater severity of lung disease.(14) Although studies have demonstrated that acute exacerbations in CF patients do not modify the coefficient of variation for lung function measured over the course of the same day,(15) other studies have shown that such exacerbations do significantly reduce spirometric values if measured over the course of a year. (16) However, there is still little information on how the variation in lung function over one year can influence the pulmonary and functional decline associated with the disease in subsequent years.
It would be useful to identify additional factors that might help predict lung function decline in the early stages of CF, given that, in many cases, FEV1 becomes abnormal only in the advanced stages of the disease. Therefore, the objective of the present study was to determine whether variation in lung function over the course of one year is associated with worse clinical outcomes and lung function decline in subsequent years.
METHODSThis was a retrospective cohort study, conducted by reviewing a secondary database. We included subjects with a diagnosis of CF, as confirmed by sweat chloride or genetic testing, who were 4-19 years of age and had been treated at the Cystic Fibrosis Outpatient Center of the São Lucas Hospital of the Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS, Pontifical Catholic University of [the state of] Rio Grande do Sul), located in the city of Porto Alegre, Brazil. During the period under study, approximately 80 CF patients were being followed at the Cystic Fibrosis Outpatient Center. Once every three months, each patient underwent clinical evaluation and pulmonary function testing, at which time samples (oropharyngeal swab or sputum samples) were collected for culture. The principal criterion for inclusion was having undergone pulmonary function testing (spirometry) at least three times in the first year (each set of tests having been performed at least three months apart) and at least once in each of the two follow-ing years. In addition, we included only individuals for whom the spirometric values were acceptable and reproduci-ble according to international guidelines, including those established for preschool-age children.(17) The variation in FEV1 (FEV1) in the first year was calculated by the following formula:
FEV1 = (hiFEV1 − loFEV1) / hiFEV1
where hiFEV1 is the highest FEV1 (% of predicted) and loFEV1 is the lowest FEV1 (% of predicted). If a subject under-went pulmonary function testing more than once in the second or third year, we selected the best spirometric result obtained, meaning the highest FEV1 (in percentage of the predicted value), in each year evaluated. We excluded subjects for whom the database contained incomplete data. The study was approved by the PUCRS Research Ethics Committee (Protocol no. 08/04102).
For each subject, we collected demographic data (age, gender, and race) and anthropometric data, as well as in-formation related to chronic infection with P. aeruginosa, number of days using antibiotics (oral. intravenous, or both), and hospitalization. Chronic P. aeruginosa infection was defined as persistent P. aeruginosa infection for at least six consecutive months (three consecutive tests), as determined by culture of oropharyngeal swab or sputum samples (depending on age or clinical status). To facilitate further analysis, antibiotic use and hospitalization were evaluated as dichotomous variables (antibiotic use, yes/no; hospitalization, yes/no). In addition, we collected data on pulmo-nary function test (spirometry) results and 6MWD. Furthermore, we determined the rate of FEV1 decline by subtracting the best third-year FEV1 from the best first-year FEV1. The final results are expressed as percentages of the predicted values. The data were entered into a database, stratified by year (first, second, and third year).
Pulmonary function tests were performed with a Koko spirometer (PDS Instrumentation, Inc., Louisville, CO, USA). The spirometric parameters evaluated included FVC, FEV1, and FEF25-75%. All procedures were performed in accordance with the criteria established by the American Thoracic Society.(17) Spirometric data are presented as percentages of the predicted values. (18) A normal FEV1 was defined as a value ≥ 80% of that predicted.
The six-minute walk test (6MWT) was performed in accordance with the American Thoracic Society guidelines.(19) The parameters evaluated in the test included heart rate; SpO2, measured with a pulse oximeter (PalmSAT 2500; Nonin Medical, Plymouth, MN, USA); blood pressure, measured with a sphygmomanometer (Tycos CE0050; Welch Allyn, Skaneateles Falls, NY, USA); respiratory rate, counted as chest wall excursions per minute; and the modified Borg scale score, to quantify the perceived intensity of dyspnea. Subjects were instructed to walk as quickly as pos-sible for six minutes in a 30-m corridor. The 6MWD was calculated by counting the total number of turns made during the test and is expressed in meters. We normalized the 6MWD using a reference equation.(20) Like FEV1, the 6MWD was considered normal if ≥ 80% of the predicted value.
Sample size was estimated based on the behavior of the main variables of interest (FEV1 and 6MWD). Adopting a level of significance of p = 0.05, a power of 80% and a minimum correlation of 0.40, we estimated the minimum sample size to be approximately 32 subjects.
Data normality was tested with the Kolmogorov-Smirnov test. Data with normal distribution are expressed as means and standard deviations. The FEV1 in the first year was calculated as described above. Because FEV1 in the first year had a skewed distribution, we applied square root transformation of the data. Categorical variables are presented as absolute and relative frequencies. In order to analyze differences in the FEV1 in the first year in rela-tion to the main clinical outcomes assessed in the two subsequent years (rate of FEV1 decline, hospitalization, 6MWD, absolute FEV1, and antibiotic use), we used the Student's t-test for independent samples. Correlations between varia-bles were assessed using the Pearson correlation test. We also used a stepwise multiple linear regression model to assess the influence that potential predictor variables (age, gender, body mass index, chronic infection with P. aeru-ginosa, absolute FEV1 at baseline, and FEV1 in the first year) had on the rate of FEV1 decline. Data were processed and analyzed with the IBM SPSS Statistics software package, version 18.0 (IBM Corporation, Armonk, NY, USA). In all tests, values of p < 0.05 were considered statistically significant.
RESULTSA total of 38 CF patients were selected for inclusion. For three patients, the data in the database were incomplete, and those patients were therefore excluded. Consequently, the final study sample comprised 35 subjects, of whom 19 (54.2%) were male. The mean age was 11.3 3.8 years. The majority of patients presented anthropometric values within the normal ranges. In general, the sample presented with mild impairment of lung function. The demographic, anthropometric, and clinical characteristics of the sample are shown in Table 1.
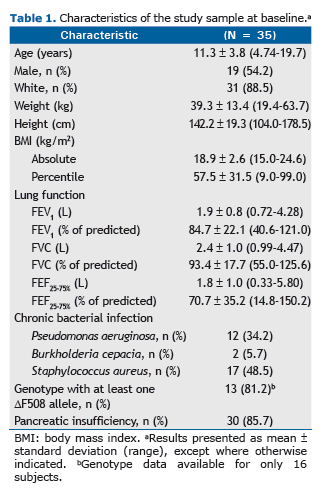
In the sample as a whole, the mean FEV1 in the first year was 0.39 0.13%. As can be seen in Figure 1A, that vari-ation was significantly greater among patients who required hospitalization in the third year than among those who did not (p = 0.03). Figure 1B shows that the mean FEV1 in the first year was also significantly greater among patients in whom the 6MWD in the third year was below normal than among those in whom it was normal (p = 0.02). In addi-tion, the mean FEV1 in the first year was significantly greater among the patients who showed lower FEV1 values in the third year (p = 0.03; Figure 1C). However, regarding the use of antibiotic therapy (Figure 1D), the mean FEV1 in the first year did not differ significantly between the patients who were treated with antibiotics in the third year and those who were not (p = 0.44).
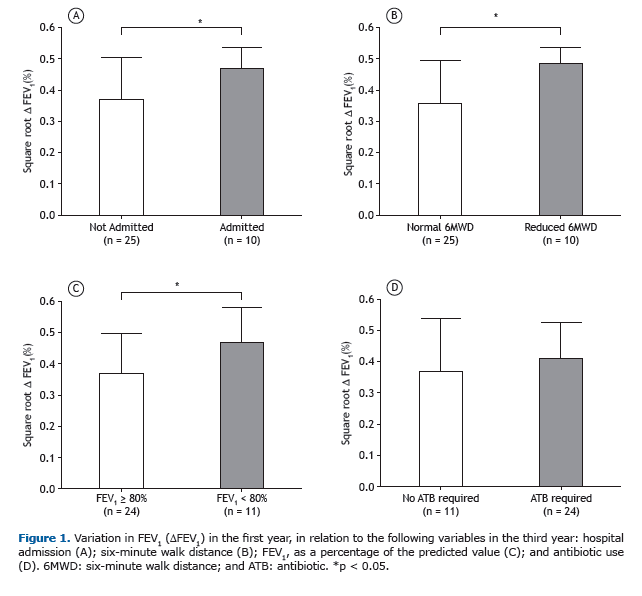
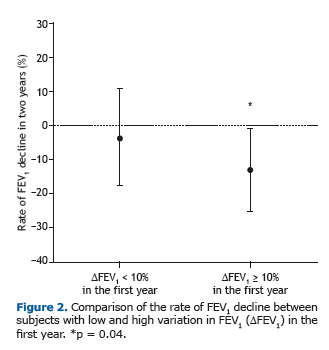
Among the patients who showed a ≥ 10% FEV1 in the first year, the rate of FEV1 decline over the two following years was significantly greater than among those who did not (p = 0.04; Figure 2). In addition, we identified a signifi-cant negative correlation between FEV1 in the first year and absolute FEV1 (% of predicted) in the third year (r = −0.340, p = 0.04), demonstrating that greater variation in lung function in the first year translated to lower FEV1 in the third year (Figure 3A). Likewise, there was a significant negative correlation between the FEV1 in the first year and the rate of FEV1 decline over the two following years (r = −0.52, p = 0.001; Figure 3B).
The stepwise multiple linear regression model, which included age, gender, body mass index, chronic infection with P. aeruginosa, FEV1 (% of predicted) at baseline, and FEV1 in the first year (Table 2), revealed that FEV1 in the first year was the only significant predictor of the rate of FEV1 decline over the two following years (p = 0.001). The model showed that FEV1 in the first year explained 27% of the subsequent rate of FEV1 decline.
 DISCUSSION
DISCUSSIONThe results of the present study suggest that, in children and adolescents with CF, a greater FEV1 over a one-year period is associated with a more pronounced decline in lung function and worse clinical outcomes over the subse-quent years. In addition, although the subjects evaluated here showed only mild impairment of lung function and preserved nutritional status, the FEV1 was found to be a predictor of progressive pulmonary decline, indicating that, even in the early stages of CF progression, quantification of this parameter can facilitate clinical detection of the disorder.
Reduced lung function, as identified by the measurement of FEV1, seems to be associated with higher mortality in CF.(12,21) However, in many cases, lung function decreases only in the advanced stages of the disease. The findings of the present study demonstrate that subjects who showed greater variation in FEV1 over a one-year period had a greater decline in lung function over the next two years of monitoring. However, the correlation was not strong, which might be explained by the fact that the study sample was composed of young subjects with preserved nutritional status and mild pulmonary impairment. In addition, our findings show that there was a moderate correlation between the FEV1 in the first year and the rate of FEV1 decline over the two following years, indicating that greater variation in lung function over a one-year period translates to a higher rate of decline in lung function in subsequent years. In a previous study,(8) FEV1 variations ≥ 13% were found to be predictive of more rapid clinical progression of lung im-pairment in CF, and lesser changes were attributed to normal fluctuations in the test. However, other studies have suggested that better lung function is associated with greater variability on the test.(22)
Subjects for whom the FEV1 in the first year was greater showed a reduction in lung function over the next two years of monitoring. That finding demonstrates that, although the determination of FEV1 is considered a useful tool for monitoring the progression of pulmonary impairment in patients with CF, calculating the FEV1 could be a comple-mentary monitoring tool, given that it could be used earlier than can the measurement of FEV1 at a single time point, because, in many cases, the latter is associated with increased mortality only in the advanced stages of the dis-ease.(12,21) In the present study, the subjects who showed a ≥ 10% FEV1 in the first year presented a more pro-nounced decline in FEV1 over the two following years.
As previously mentioned, the multiple linear regression model showed that 27% of the rate of FEV1 decline over the next two years of monitoring could be explained by the FEV1 during the first year. This result highlights the im-portance of assessing the variation in lung function over a relatively short period of time, given the observed de-crease in lung function thereafter. Therefore, we believe that such assessment can represent an additional, useful tool for monitoring disease progression in CF, because an isolated reduction in FEV1 is often seen only in the advanced stages of the disease. Nevertheless, when analyzing the data on variability, we found that approximately 46% of our subjects had a low baseline FEV1, with a consequent increase in FEV1 over the first year, showing that this parameter indicates the variability in lung function in general, rather than specifically indicating the progressive decline ex-pected in CF.
On the basis of a recent review of the literature, we believe that this is the first study to show that short-term varia-tion in lung function is associated with worse clinical outcomes over time in CF patients. Our findings demonstrate that subjects who showed greater FEV1 in the first year were more likely to require hospitalization in the third year. These findings corroborate those of previous studies showing that pulmonary exacerbations cause a decline in lung function over time and that a decline in FEV1 is associated with the severity of pulmonary exacerbations, requiring hospitalization and intravenous administration of antibiotics.(7,23,24) In addition, a decrease in FEV1 seems to be a predictor of hospitalization and mortality in CF,(6,13) and we found that FEV1 over a one-year period had a similar relationship with CF outcomes in the present study.
Previous studies have found an association between the use of antibiotics and a decline in lung function in patients with CF.(7,24) In the present study, we found no statistically significant correlation between FEV1 in the first year and antibiotic use. That might be attributable to the small size of our sample and the short study period. Other authors have shown that the use of intravenous antibiotics to treat pulmonary exacerbations is a risk factor for the decline in lung function over time in CF patients.(23,25) In addition, it has been suggested that consecutive exacerbations over a short period of time contribute to the progression of lung disease. Furthermore, one previous study demonstrated that the occurrence of three pulmonary exacerbations per year increases the risk of a decline in FEV1 by approximately 5%.(26)
The 6MWT is featured as an important tool for the functional assessment of individual responses to exercise, provid-ing a comprehensive analysis of the cardiovascular and pulmonary function, in the general population as well as in individuals with CF.(19,27) Impaired lung function, malnutrition, and muscle weakness have been described as playing major roles in determining the physical performance of CF patients. In addition, a higher respiratory rate, with re-duced tidal volume ventilation and hypoxemia, also seems to limit physical activity.(27,28) Recent studies have shown that there is a significant correlation between the 6MWD and other important clinical outcomes, such as FEV1, FVC, and disease severity, in CF patients.(14,29,30) In our study, subjects who showed a greater FEV1 in the first year also presented a below-normal 6MWD in the third year, indicating that the calculation of FEV1 can be an important tool for predicting functional worsening in CF patients. Although two equations have been devised for standardizing 6MWD values in Brazil,(31,32) we chose to use international reference values,(20) because the latter include the entire age range represented in our sample and were generated from White individuals, which is relevant given that the majori-ty of the patients in our sample were White.
Our study has certain limitations, primarily those that are inherent to the use of a retrospective design and data col-lection based on searches of secondary databases. In addition, our sample was quite homogeneous in terms of lung function and nutritional status.
In summary, our findings suggest that variation in lung function over a one-year period is associated with a higher rate of FEV1 decline and worse clinical outcomes in subsequent years. Assessing the FEV1 over a relatively short period of time could, in conjunction with routine monitoring of FEV1, contribute to the prediction of disease progres-sion. Therefore, the calculation of this parameter might become an additional tool for more careful monitoring of the clinical progression of lung disease in patients with CF.
REFERENCES1. Beker LT, Russek-Cohen E, Fink RJ. Stature as a prognostic factor in cystic fibrosis survival. J Am Diet Assoc. 2001;101(4):438-42. http://dx.doi.org/10.1016/S0002-8223(01)00113-4
2. Duguépéroux I, Tamalet A, Sermet-Gaudelus I, Le Bourgeois M, Gérardin M, Desmazes-Dufeu N, et al. Clinical changes of patients with cystic fibrosis during transition from pediatric to adult care. J Adolesc Health. 2008;43(5):459-65. http://dx.doi.org/10.1016/j.jadohealth.2008.03.005
3. Southern KW, Munck A, Pollitt R, Travert G, Zanolla L, Dankert-Roelse J, et al. A survey of newborn screening for cystic fibrosis in Europe. J Cyst Fibros. 2007;6(1):57-65. http://dx.doi.org/10.1016/j.jcf.2006.05.008
4. Que C, Cullinan P, Geddes D. Improving rate of decline of FEV1 in young adults with cystic fibrosis. Thorax. 2006;61(2):155-7. http://dx.doi.org/10.1136/thx.2005.043372
5. Dodge JA, Lewis PA, Stanton M, Wilsher J. Cystic fibrosis mortality and survival in the UK: 1947-2003. Eur Respir J. 2007;29(3):522-6. http://dx.doi.org/10.1183/09031936.00099506
6. Liou TG, Elkin EP, Pasta DJ, Jacobs JR, Konstan MW, Morgan WJ, et al. Year-to-year changes in lung function in individuals with cystic fibrosis. J Cyst Fibros. 2010;9(4):250-6. http://dx.doi.org/10.1016/j.jcf.2010.04.002
7. Konstan MW, Morgan WJ, Butler SM, Pasta DJ, Craib ML, Silva SJ, et al. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J Pediatr. 2007;151(2):134-9, 139.e1.
8. Taylor-Robinson D, Whitehead M, Diderichsen F, Olesen HV, Pressler T, Smyth RL, et al. Understanding the natural progression in %FEV1 decline in patients with cystic fibrosis: a longitudinal study. Thorax. 2012;67(10):860-6. http://dx.doi.org/10.1136/thoraxjnl-2011-200953
9. Goss CH, MacNeill SJ, Quinton HB, Marshall BC, Elbert A, Knapp EA, et al. Children and young adults with CF in the USA have better lung function compared with the UK. Thorax. 2015;70(3):229-36. http://dx.doi.org/10.1136/thoraxjnl-2014-205718
10. Mannino DM, Reichert MM, Davis KJ. Lung function decline and outcomes in an adult population. Am J Respir Crit Care Med. 2006;173(9):985-90. http://dx.doi.org/10.1164/rccm.200508-1344OC
11. Konstan MW, Wagener JS, Yegin A, Millar SJ, Pasta DJ, VanDevanter DR. Design and powering of cystic fibrosis clinical trials using rate of FEV(1) decline as an efficacy endpoint. J Cyst Fibros. 2010;9(5):332-8. http://dx.doi.org/10.1016/j.jcf.2010.05.004
12. Corey M, Edwards L, Levison H, Knowles M. Longitudinal analysis of pulmonary function decline in patients with cystic fibrosis. J Pediatr. 1997;131(6):809-14. http://dx.doi.org/10.1016/S0022-3476(97)70025-8
13. Konstan MW, Wagener JS, Vandevanter DR, Pasta DJ, Yegin A, Rasouliyan L, et al. Risk factors for rate of decline in FEV1 in adults with cystic fibrosis. J Cyst Fibros. 2012;11(5):405-11. http://dx.doi.org/10.1016/j.jcf.2012.03.009
14. Stollar F, Rodrigues JC, Cunha MT, Leone C, Adde FV. Six minute walk test Z score: correlations with cystic fibrosis severity markers. J Cyst Fibros. 2012;11(3):253-6. http://dx.doi.org/10.1016/j.jcf.2011.11.009
15. Sanders DB, Rosenfeld M, Mayer-Hamblett N, Stamey D, Redding GJ. Reproducibility of spirometry during cystic fibrosis pulmonary exacerbations. Pediatr Pulmonol. 2008;43(11):1142-6. http://dx.doi.org/10.1002/ppul.20924
16. Mayer OH, Jawad AF, McDonough J, Allen J. Lung function in 3-5-year-old children with cystic fibrosis. Pediatr Pulmonol. 2008;43(12):1214-23. http://dx.doi.org/10.1002/ppul.20930
17. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of Spirometry. Eur Resp J. 2005;26(2):319-38. http://dx.doi.org/10.1183/09031936.05.00034805
18. Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324-43. http://dx.doi.org/10.1183/09031936.00080312
19. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111-7. http://dx.doi.org/10.1164/ajrccm.166.1.at1102
20. Geiger R, Strasak A, Treml B, Gasser K, Kleinsasser A, Fischer V, et al. Six-minute walk test in children and adolescents. J Pediatr. 2007;150(4):395-9, 399.e1-2.
21. Milla CE. Association of nutritional status and pulmonary function in children with cystic fibrosis. Curr Opin Pulm Med. 2004;10(6):505-9. http://dx.doi.org/10.1097/01.mcp.0000138995.08494.69
22. Enright PL, Beck KC, Sherrill DL. Repeatability of spirometry in 18,000 adult patients. Am J Respir Crit Care Med. 2004;169(2):235-8. http://dx.doi.org/10.1164/rccm.200204-347OC
23. Waters V, Stanojevic S, Atenafu EG, Lu A, Yau Y, Tullis E, et al. Effect of pulmonary exacerbations on long-term lung function decline in cystic fibrosis. Eur Respir J. 2012;40(1):61-6. http://dx.doi.org/10.1183/09031936.00159111
24. Amadori A, Antonelli A, Balteri I, Schreiber A, Bugiani M, De Rose V. Recurrent exacerbations affect FEV(1) decline in adult patients with cystic fibrosis. Respir Med. 2009;103(3):407-13. http://dx.doi.org/10.1016/j.rmed.2008.09.024
25. Sanders DB, Bittner RC, Rosenfeld M, Redding GJ, Goss CH. Pulmonary exacerbations are associated with subsequent FEV1 decline in both adults and children with cystic fibrosis. Pediatr Pulmonol. 2011;46(4):393-400. http://dx.doi.org/10.1002/ppul.21374
26. de Boer K, Vandemheen KL, Tullis E, Doucette S, Fergusson D, Freitag A, et al. Exacerbation frequency and clinical outcomes in adult patients with cystic fibrosis. Thorax. 2011;66(8):680-5. http://dx.doi.org/10.1136/thx.2011.161117
27. Pereira FM, Ribeiro MÂ, Ribeiro AF, Toro AA, Hessel G, Ribeiro JD. Functional performance on the six-minute walk test in patients with cystic fibrosis. J Bras Pneumol. 2011;37(6):735-44.
28. Thin AG, Dodd JD, Gallagher CG, Fitzgerald MX, Mcloughlin P. Effect of respiratory rate on airway deadspace ventilation during exercise in cystic fibrosis. Respir Med. 2004;98(11):1063-70. http://dx.doi.org/10.1016/j.rmed.2004.03.016
29. Ziegler B, Rovedder PM, Lukrafka JL, Oliveira CL, Menna-Barreto SS, Dalcin Pde T. Submaximal exercise capacity in adolescent and adult patients with cystic fibrosis. J Bras Pneumol. 2007;33(3):263-9. http://dx.doi.org/10.1590/S1806-37562007000300006
30. Stollar F, Adde FV, Cunha MT, Leone C, Rodrigues JC. Shwachman-Kulczycki score still useful to monitor cystic fibrosis severity. Clinics (Sao Paulo). 2011;66(6):979-83. http://dx.doi.org/10.1590/S1807-59322011000600010
31. Priesnitz CV, Rodrigues GH, Stumpf Cda S, Viapiana G, Cabral CP, Stein RT, et al. Reference values for the 6-min walk test in healthy children aged 6-12 years. Pediatr Pulmonol. 2009;44(12):1174-9. http://dx.doi.org/10.1002/ppul.21062
32. Iwama AM, Andrade GN, Shima P, Tanni SE, Godoy I, Dourado VZ. The six-minute walk test and body weight-walk distance product in healthy Brazilian subjects. Braz J Med Biol Res. 2009;42(11):1080-5. http://dx.doi.org/10.1590/S0100-879X2009005000032








