ABSTRACT
Objective: To evaluate the impact of lipoabdominoplasty on diaphragmatic mobility (DM) and lung function in healthy women. Methods: This was a prospective cohort study using high-resolution ultrasound and forced spirometry to assess DM and lung function, respectively, prior to lipoabdominoplasty, as well as on postoperative day (POD) 10 and POD 30. DM was measured under two conditions: during tidal volume breathing and during a VC maneuver. Results: The sample consisted of 20 women, with a mean age of 39.85 ± 7.52 years and a mean body mass index of 26.21 ± 2.0 kg/m2. Comparing the preoperative and postoperative periods, we found that DM and lung function values were significantly lower after lipoabdominoplasty, the mean DM on POD 10 being 17% and 15% lower during tidal volume breathing and during the VC maneuver, respectively, in comparison with the preoperative mean (p = 0.009 and p < 0.001, respectively). In addition, FEV1, FVC, and PEF were significantly lower on POD 10 than in the preoperative period (p = 0.046, p = 0.002, and p < 0.001, respectively), returning to preoperative values by POD 30. Conclusions: Lipoabdominoplasty appears to have negative short-term repercussions for DM and lung function in healthy women. However, lung function and DM are both apparently restored to preoperative conditions by POD 30.
(ClinicalTrials.gov identifier: NCT02762526 [http://www.clinicaltrials.gov/])
Keywords:
Abdominoplasty; Lipectomy; Ultrasonography; Diaphragm/physiology; Diaphragm/physiopathology; Lung/physiology; Lung/physiopathology; Respiratory mechanics; Spirometry; Respiratory function tests.
RESUMO
Objetivo: Avaliar o impacto da lipoabdominoplastia na mobilidade diafragmática (MD) e na função pulmonar de mulheres saudáveis. Métodos: Estudo prospectivo de coorte com ultrassonografia de alta resolução e espirometria forçada para a avaliação da MD e da função pulmonar, respectivamente, antes da lipoabdominoplastia, no 10º dia do pós-operatório e no 30º dia do pós-operatório. A MD foi medida durante a respiração em volume corrente e durante uma manobra de CV. Resultados: A amostra foi composta por 20 mulheres, com média de idade de 39,85 ± 7,52 anos e média de índice de massa corporal de 26,21 ± 2,0 kg/m2. Ao compararmos os períodos pré e pós-operatório, observamos que a MD e a função pulmonar foram significativamente menores após a lipoabdominoplastia; a média de MD no 10º dia do pós-operatório foi 17% menor durante a respiração em volume corrente e 15% menor durante a manobra de CV do que a média pré-operatória (p = 0,009 e p < 0,001, respectivamente). Além disso, o VEF1, a CVF e o PFE foram significativamente menores no 10º dia do pós-operatório que no pré-operatório (p = 0,046, p = 0,002 e p < 0,001, respectivamente), retornando aos valores pré-operatórios até o 30º dia do pós-operatório. Conclusões: A lipoabdominoplastia parece ter repercussões negativas em curto prazo na MD e função pulmonar de mulheres saudáveis. No entanto, tanto a função pulmonar como a MD aparentemente retornam ao estado pré-operatório até o 30º dia do pós-operatório.
(ClinicalTrials.gov identifier: NCT02762526 [http://www.clinicaltrials.gov/])
Palavras-chave:
Abdominoplastia; Lipectomia; Ultrassonografia; Diafragma/fisiologia; Diafragma/fisiopatologia; Pulmão/fisiologia; Pulmão/fisiopatologia; Mecânica respiratória; Espirometria; Testes de Função Respiratória.
INTRODUCTIONPlastic surgery techniques for contouring the abdomen, such as lipoabdominoplasty, are among the most often requested procedures, ranking third among plastic surgery techniques performed worldwide. (1-4) Lipoabdominoplasty, which combines classical abdominoplasty and liposuction, results in a significant reduction in the fat pad and has the added benefits of muscle plication and the removal of skin tissue.(5) It has become common practice among plastic surgeons and has a low incidence of postoperative complications if the clinical condition of the patient is evaluated prior to the procedure.(5,6) However, there have been reports of respiratory comorbidities, including respiratory failure, atelectasis, pneumonia, and bronchospasm, in the postoperative period after lipoabdominoplasty.(7-11) Such complications might be attributable to increased intra-abdominal pressure (IAP), caused by plication of the aponeurosis of the rectus abdominis muscle, which could lead to changes in diaphragmatic mobility (DM),(12) as well as impaired lung function.(10,13) In some of the cases reported, lung function was reduced to half of that observed prior to surgery. Although none of the authors of those reports evaluated DM in the affected patients, other factors have been implicated in a postoperative decrease in lung volumes, such as the administration of anesthetic drugs, visceral manipulation, the incision in the abdominal wall, and patient fear of injury from the surgery.(6,14) Pain exerts an effect on the postoperative evolution of patients submitted to abdominal surgery, with a negative impact on lung function.(15)
The primary objective of this study was to identify respiratory complications in healthy women undergoing lipoabdominoplasty. To that end, we evaluated DM and lung function at several time points.
METHODSStudy design and participantsThis was a prospective cohort study conducted in the Physical Therapy Sector of the Laboratory of Physiology and Cardiopulmonary Physiotherapy and at the Plastic Surgery Clinic of the Hospital das Clínicas da Universidade Federal de Pernambuco (HC-UFPE, Federal University of Pernambuco Hospital das Clínicas), as well as at the Plastic Surgery Clinic of the Hospital Agamenon Magalhães, between July of 2015 and March of 2016. The study was approved by the Human Research Ethics Committee of the HC-UFPE Health Sciences Center (CAAE protocol no. 15225913.0.0000.5208) and was registered with ClinicalTrials.gov (identifier: NCT02762526). We employed a non-probability sampling process, in which we screened all individuals who met the eligibility criteria.
Eligibility criteriaWe included women between 25 and 55 years of age who underwent lipoabdominoplasty with plication of the rectus abdominis muscle. All had type IV or V abdominal deformity, as described by Bozola.(16) We selected women with no history of respiratory or cardiac comorbidities. We also selected only women with a body mass index ≤ 30 kg/m2 and a minimum score of 18 on the Mini-Mental State Examination.
We excluded women who were current smokers or had a smoking history greater than 10 pack-years. Women with an FEV1 < 80% of the predicted value or an FEV1/FVC ratio < 70% of the predicted value were also excluded.
Surgical procedureFor the surgical procedure, all patients were sedated and received spinal or epidural anesthesia. First, the surgical site was marked. A solution of epinephrine (diluted 1:250,000 in saline) was then infiltrated into the abdominal cavity to initiate liposuction. The aspiration began through the supra-umbilical region, continuing along the sides and through the infra-umbilical region. After the liposuction, the navel was isolated and only the infra-umbilical skin was resected, as in classic abdominoplasty.(5)
Umbilicoplasty was performed by attaching the deep dermis of the navel to the aponeurosis of the rectus abdominis muscle, after which the deep dermis of the neo-umbilicus was attached to the flap with monocryl 3-0 sutures at the intercardinal points. The skin flap was fixed in two planes, with mononylon 3-0 sutures in the subcutaneous tissue and monocryl 5-0 sutures in the dermis, initially with separate single sutures and subsequently with continuous sutures. At the intercardinal and cardinal points of the navel, respectively, we used the modified Allgöwer-Donati suture and single sutures, both with mononylon 4-0 sutures.(17)
Outcome measuresAll of the outcomes investigated were measured at three time points: in the preoperative period, on postoperative day (POD) 10, and on POD 30. Initially, medical histories were taken and all of the patients underwent a physical examination. Personal data were collected, and we recorded anthropometric measurements-weight (in kg), height (in m), and body mass index (in kg/m2)-as well as vital signs-HR, SpO2, RR, and blood pressure. Patients were asked to remain seated with their arms resting on their legs and to remain quiet during the measurement of vital signs.
DMWe used a high-resolution ultrasound system (SonoAce R3; Samsung Medison, Seoul, South Korea) with a convex 3.5 MHz transducer. The protocol used was that suggested by Testa et al.(12,18) The patients received a verbal command to breathe evenly for the measurement of DM during tidal volume (VT) breathing and then to perform VC maneuvers (Figure 1), during which each curve relating to the displacement of the dome of the diaphragm (in mm) was measured immediately after acquisition of the images. The maneuvers were repeated in order to obtain five satisfactory images. We considered the average of the three highest values that were within 10% of each other.
 Lung function
Lung functionWe used a portable spirometer (Microloop MK8; Micro Medical, Kent, England) in order to evaluate FVC, FEV1, FEF between 25% and 75% of the FVC (FEF25-75%), PEF, and the FEV1/FVC ratio. The maneuvers were carried out in accordance with the recommendations of the American Thoracic Society(19) and other guidelines for pulmonary function testing.(20)
DyspneaPatients were asked about their perception of dyspnea at rest and during the procedures performed. We applied the modified Borg Scale, in accordance with the American Thoracic Society recommendations.(19)
PainWe used a visual analog scale(21) consisting of a one-dimensional instrument for graduation of pain intensity level. Pain was assessed at rest during each respiratory evaluation. To avoid measurement bias, all procedures were performed by the same examiner during all phases of the study.
Statistical analysisThe sample size was calculated, on the basis of the results of a pilot study involving 10 patients, with the G*Power3 software package.(22) The calculation was performed by determining the mean difference between the preoperative and POD 10 values (∆1), as well as between the preoperative and POD 30 values (∆2) for the most clinically relevant variables: FEV1, FVC, and DM. For FEV1, we observed a ∆1 value of 13.4 ± 5 and a ∆2 value of 3.5 ± 3. For FVC, we observed a ∆1 value of 11.8 ± 2.62 and a ∆2 value of 4.7 ± 4.5. For DM, we observed a ∆1 value of 18 ± 7.16 and a ∆2 value of 2.62 ± 3.13. Therefore, we required 5 patients for FEV1, 7 patients for FVC, and 7 patients for DM, which would give all of those variables a power of 95% and an alpha of 0.05. However, we decided to select 27 patients, considering the potential for losses to follow-up in prospective cohort studies.
The data were analyzed with SigmaPlot software for Windows, version 12.0 (Systat Software, San Jose, CA, USA). To characterize the sample, we calculated descriptive statistics, using means ± standard deviations, means (95% CIs), or medians (interquartile ranges) for quantitative variables.
The Shapiro-Wilk test and Levene's test for homogeneity of variance were applied in order to verify the normality and homogeneity of the data, respectively. For comparisons among time points, we used two-way repeated-measures ANOVA with the Holm-Sidak post hoc test to compare means for quantitative variables with normal, homogeneous distribution. For quantitative variables with a non-normal distribution, we used Friedman's repeated-measures ANOVA on ranks, followed by Tukey's post hoc test for variables that presented a statistical difference. The effect size was determined by calculating the Cohen's d, which involved obtaining the mean difference between the time points and dividing the result by the pooled standard deviation.(23) Pearson's correlation coefficient was used in order to detect associations between DM variables and lung function variables. Statistical analyses were performed with the IBM SPSS Statistics software package, version 20.0 (IBM Corporation, Armonk, NY, USA). Values of p < 0.05 were considered statistically significant.
RESULTSThe initial sample comprised 27 women, all of whom underwent lipoabdominoplasty. However, only 20 of those women completed the protocol (Figure 2). Anthropometric variables, clinical characteristics, and vital signs are summarized in Table 1. Two patients were excluded from the analysis of DM, because of technical problems related to the ultrasound examination. On POD 10, DM was relatively low, during VT breathing and at TLC-9.54 mm (8.42-10.99 mm) and 51.23 mm (41.66-55.89 mm), respectively-those values returning to normal by POD 30-12.37 mm (10.49-14.13 mm) and 63.35 mm (55.19-68.34 mm), respectively-at which point they were comparable to those obtained in the preoperative period-11.56 mm (9.65-13.48 mm) and 60.15 mm (51.95-67.84 mm), respectively. As can be seen in Figure 3, the differences between the preoperative and postoperative values were significant for the images acquired during VT breathing (p = 0.009), as well as for those acquired during the VC maneuver (p < 0.001).


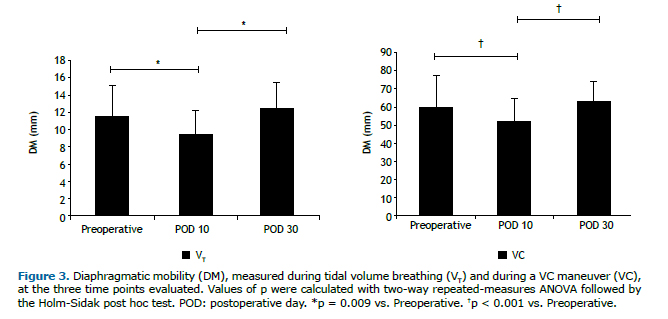
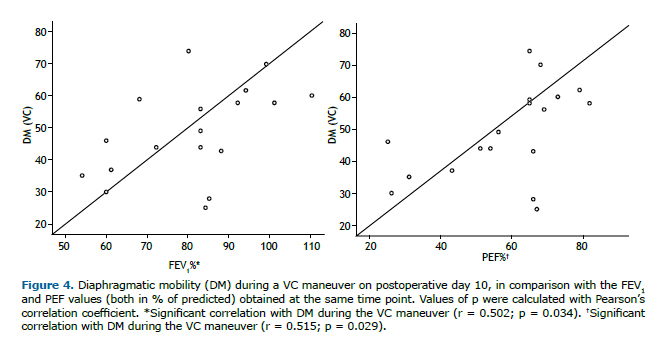
Lung function parameters at the three time points evaluated are shown in Table 2. There were significant differences among the time points for FEV1, FVC, and PEF, all of which were lower on POD 10 than in the preoperative period and were restored to normal values by POD 30. The FEV1/FVC ratio and FEF25-75% remained unchanged after lipoabdominoplasty, with no statistical differences between any of the time points. On POD 10, the DM measured during the VC maneuver correlated positively, albeit moderately, with FEV1 (r = 0.502; p = 0.034) and PEF (r = 0.515; p = 0.029), as depicted in Figure 4.

Of the 20 women evaluated, 7 reported pain on POD 10. Three of those women classified their pain as mild and 4 classified it as moderate. One of the women reported moderate pain on POD 30. Two women reported perceived dyspnea on POD 10, the dyspnea being classified as not very intense in one case and very intense in the other. On POD 30, none of the patients reported dyspnea. No differences were observed between the pain/dyspnea reported at rest and that reported during the execution of the maneuvers, in terms of the perception or intensity of pain or in terms of the perception of dyspnea, at any of the time points evaluated.
DISCUSSIONThe main findings of our study are that lipoabdominoplasty with plication of the rectus abdominis muscle promoted a reduction in DM and worsening of lung function in healthy women, as evaluated on POD 10. However, both of those parameters showed a tendency to return to preoperative values by POD 30.
In comparison with the preoperative means, the mean DM on POD 10 was 17% lower when measured during VT breathing and 15% lower when measured during the VC maneuver. By POD 30, the DM had returned to values comparable to those obtained in the preoperative period. However, there is a shortage of studies evaluating DM in this specific population, which precludes any comparisons with our findings.
We hypothesize that there were two main causes of the behavior of DM in our sample. The first potential cause was the influence of plication of the rectus abdominis muscle near the xiphoid process, and the second was the increase in IAP.
Regarding the influence of plication of the rectus abdominis muscle, we suggest that approximation of the edges of the rectus abdominis muscles can generate a higher tensile strength in the fibers of those muscles, reducing the anteroposterior and transverse diameter of the chest, which will in turn reduce DM, because of the anatomical proximity between the upper insertions of the rectus abdominis muscles and one of the origins of the diaphragm muscle. The rectus abdominis muscle attaches to the fifth, sixth, and seventh costal cartilages and to the xiphoid process, whereas the costal portion of the diaphragm also originates from the last six ribs. In addition, the trunk flexion posture adopted by the patient for just over 10 days after the procedure can increase the magnitude of the changes.(24,25)
The primary action of the transversus abdominis and abdominal oblique muscles is to pull the abdominal wall inward, increasing the IAP. In doing so, they induce cranial displacement of the diaphragm, leading to an increase in pleural pressure and a consequent reduction in lung volume. Although the rectus abdominis muscle must do the same when the ventral abdominal wall has an outward convexity, we would expect that when the convexity is inward, an isolated muscle contraction would pull the wall slightly outward.(26) In an animal study, De Troyer et al.(27) analyzed the selective activation of the rectus abdominis muscle by electrical stimulation, finding that the ribcage and sternum were displaced in caudal and anteroposterior directions, whereas the transverse diameters of the lower ribcage were decreased. Therefore, the plication of the rectus abdominis muscle causes an increase in IAP and pleural pressure, regardless of the shape of the abdominal wall. Despite the limitations of comparison, these findings corroborate the hypothesis put forth in our study: that plication of the aponeurosis generates tension and thus limits thoracic expansion. We hypothesize that the reduction in DM can also be explained by the postoperative increase in IAP, which persisted at least until POD 10, caused by the plication of the rectus abdominis muscle to correct diastasis recti, which prevents the diaphragm from descending.(26) After plication, the abdominal region can properly assist in the lung expansion and IAP values remain within the normal range, which in healthy adults is up to 5 mmHg. Pressures above 15 mmHg can cause further damage to the respiratory system.(28,29) Although our study does not present the IAP values of the women who underwent lipoabdominoplasty, other authors have described the behavior of IAP after similar surgical procedures. Talisman et al.(30) measured IAP oscillations during abdominoplasty in 18 patients and studied the relevance of such oscillations for patient evolution in the immediate postoperative period. Three patients who underwent plication to correct diastasis recti presented an IAP above 24 cmH2O in the immediate postoperative period and above 20 cmH2O on POD 1. The authors concluded that such patients are at a higher risk of developing respiratory distress in the immediate postoperative period.
Plication of the rectus abdominis muscle near the xiphoid process and the consequent increase in IAP would place the diaphragm at a mechanical disadvantage, resulting in restrictive lung disease in patients undergoing this type of surgery. In our patient sample, we observed a postoperative decrease in spirometric parameters, the lower values persisting at least until POD 10.
The spirometric data obtained in our study show that there was pronounced worsening of lung function on POD 10, as evidenced by lower FEV1 and FVC values, and a near-total restoration of normal lung function by POD 30. Similarly, PEF was reduced on POD 10 and returned to preoperative values on POD 30, the difference between POD 10 and POD 30 being significant. During the study period, there were no significant changes in FEF25-75% or in the FEV1/FVC ratio. These data suggest that lipoabdominoplasty promotes the development of restrictive lung disease.
To our knowledge, there have been no previous studies evaluating lung function in women undergoing lipoabdominoplasty. However, other authors have studied the behavior of lung function in the postoperative period after abdominoplasty and have observed that lung function worsens in the immediate postoperative period and returns to preoperative levels by POD 30.(10,13,31,32) Our choice to evaluate patients at that time point was based on those studies. Tercan et al.(10) evaluated 14 healthy women who underwent abdominoplasty, observing a significant decline in FVC on POD 10 and subsequent improvement by POD 30, when FVC surpassed the preoperative values, suggesting that correction of diastasis recti abdominis promotes effective containment of the abdominal wall, improving the spirometric parameters over a period > 30 days. Similarly, Helene Junior et al.(13) found that, among patients undergoing abdominoplasty, the values of FEV1, FVC, FEF25-75%, and PEF were lower on POD 4 than in the preoperative period, the FEV1/FVC ratio remaining constant, although FEV1 and FVC were both below normal, suggesting a restrictive pattern. The authors also found that FVC and PEF showed significant improvement from POD 4 to POD 15, as well as from POD 15 to POD 30, although neither returned to the preoperative value. In one long-term study of 24 patients undergoing total abdominoplasty, Perin et al.(31) evaluated spirometric parameters during the preoperative period and after a mean period of 28 months. The authors found no difference between those two time points in terms of the lung function of the patients. Rodrigues et al.(32) studied the respiratory function of patients who underwent plication of the aponeurosis of the external abdominal oblique muscle and correction of diastasis recti. Those authors observed a ventilatory pattern similar to what occurs in the postoperative period in patients undergoing only correction of diastasis recti, concluding that the use of an L-shaped plication, per se, does not increase IAP; that is, the plication is not responsible for the impairment of lung function after abdominoplasty. The authors attributed the significant postoperative increase in IAP to the use of a compression garment, citing that as the most detrimental factor.
In the present study, we found that, on POD 10, DM during the VC maneuver correlated with lung function. We looked for correlations at that time point because we believe that patients undergoing lipoabdominoplasty show the most limitations in the first 10 days after the procedure. In our patient sample, DM during the VC maneuver showed a moderate positive correlation with PEF and with FEV1, although DM during VT breathing did not correlate with any of the spirometric parameters. These data indicate that some of the limitations in lung function in these patients can be explained by the reduction in DM caused by plication, that reduction being more pronounced at maximum effort, given that the sample consisted of healthy women. It is likely that there would be a strong correlation between lung function and DM, including DM during VT breathing, in a population with pre-existing comorbidities.
In our study, pain, as measured with a visual analog scale, was reported by 35% and 5% of the patients on POD 10 and POD 30, respectively. Although some studies have shown that postoperative pain can be related to reduced lung volumes,(33) we found no correlation between pain and lung function. We also did not consider pain a relevant factor for diaphragmatic dysfunction, because the behavior of spirometric parameters and DM in the patients with pain was similar to that observed in those without. That might be due to the fact that only 7 women reported pain, probably because most of the patients received analgesic medications, as is common practice in the postoperative period. In addition, unlike what occurs during upper abdominal surgery, there is no disruption of muscle fibers during lipoabdominoplasty, which is an important distinction, because muscle injury is the main cause of postoperative pain.(34)
On POD 10, the patients in our sample showed reductions in DM and lung function. However, dyspnea was not an important clinical factor in our study, being reported by only 10% of the patients evaluated.
Our study has some limitations, such as the fact that we did not evaluate respiratory muscle strength or diaphragm thickness. There is therefore a need for further studies involving such analyses, which could better elucidate our findings.
REFERENCES1. Santos NP Dos, Barnabé AS, Fornari JV, Ferraz RRN. Pain assessment in patients undergoing cosmetic or reconstructive plastic surgery [Article in Portuguese]. Rev Bras Cir Plast. 2012;27(2):190-4.
2. American Society of Plastic Surgeons [homepage on the Internet]. Arlington Heights (IL): the Society; c2008 [cited 2017 Nov 1]. Report of the 2007 statistics: National Clearinghouse of Plastic Surgery Statistics. Available from: www.plasticsurgery.org/
3. Rohrich RJ, Stuzin JM. Globalization of plastic surgery: the world of plastic and reconstructive surgery in Brazil. Plast Reconstr Surg. 2012;130(4):967-8. https://doi.org/10.1097/PRS.0b013e31826703b1
4. Nahas FX. A pragmatic way to treat abdominal deformities based on skin and subcutaneous excess. Aesthetic Plast Surg. 2001;25(5):365-71. https://doi.org/10.1007/s00266-001-0025-7
5. Saldanha OR, Pinto EBS, Matos Jr WN, Lucon RL, Magalhães F, Bello EML, et al. Lipoabdominoplasty - Saldanha's Technique. Rev Bras Cir Plast. 2003;(2):37-46.
6. Assumpção GG. Mini-abdominoplasty associated with liposuction and lowering of the umbilical scar without pedicular detachment [Article in Portuguese]. Rev Bras Cir Plast. 2012;27(3):450-6. https://doi.org/10.1590/S1983-51752012000300021
7. ROE BB. Prevention and treatment of respiratory complications in surgery. N Engl J Med. 1960;263:547-50. https://doi.org/10.1056/NEJM196009152631106
8. Palmon SC, Kirsch JR, Depper JA, Toung TJ. The effect of the prone position on pulmonary mechanics is frame-dependent. Anesth Analg. 1998;87(5):1175-80.
9. Rezaiguia S, Jayr C. Prevention of respiratory complications after abdominal surgery [Article in French]. Ann Fr Anesth Reanim. 1996;15(5):623-46. https://doi.org/10.1016/0750-7658(96)82128-9
10. Tercan M, Bekerecioglu M, Dikensoy O, Kocoglu H, Atik B, Isik D, et al. Effects of abdominoplasty on respiratory functions: a prospective study. Ann Plast Surg. 2002;49(6):617-20. https://doi.org/10.1097/00000637-200212000-00011
11. Pierri A, Munegato G, Carraro L, Zaccaria F, Tiso E, Zotti EF. Hemodynamic alterations during massive incisional hernioplasty. J Am Coll Surg. 1995;181(4):299-302.
12. Yamaguti WP, Paulin E, Shibao S, Kodaira S, Chammas MC, Carvalho CR. Ultrasound evaluation of diaphragmatic mobility in different postures in healthy subjects. J Bras Pneumol. 2007;33(4):407-13. https://doi.org/10.1590/S1806-37132007000400009
13. Helene Junior A, Saad Junior R, Stirbulov R. Avaliação da função respiratória em indivíduos submetidos à abdominoplastia. Rev Col Bras Cir. 2006;33(1):45-50. https://doi.org/10.1590/S0100-69912006000100011
14. Grams ST, von Saltiél R, Mayer AF, Schivinski CI, de S Nobre LF, Nóbrega IS, et al. Assessment of the reproducibility of the indirect ultrasound method of measuring diaphragm mobility. Clin Physiol Funct Imaging. 2014;34(1):18-25. https://doi.org/10.1111/cpf.12058
15. Tonella RM, Araújo S, Silva AM. Transcutaneous electrical nerve stimulation in the relief of pain related to physical therapy after abdominal surgery [Article in Portuguese]. Rev Bras Anestesiol. 2006;56(6):630-42.
16. Bozola AR. Abdominoplasty: same classification and a new treatment concept 20 years later. Aesthetic Plast Surg. 2010;34(2):181-92. https://doi.org/10.1007/s00266-009-9407-z
17. Pigossi N, Tariki JY, de Cássia H, Calonge F, de Andrade AC, Misawa HT, et al. Tactics in the umbilical approach in abdominoplasties [Article in Portuguese]. Rev Hosp Clin Fac Med Sao Paulo. 1991;46(3):145-7.
18. Testa A, Soldati G, Giannuzzi R, Berardi S, Portale G, Gentiloni Silveri N. Ultrasound M-mode assessment of diaphragmatic kinetics by anterior transverse scanning in healthy subjects. Ultrasound Med Biol. 2011;37(1):44-52. https://doi.org/10.1016/j.ultrasmedbio.2010.10.004
19. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111-7. Erratum in: Am J Respir Crit Care Med. 2016;193(10):1185. https://doi.org/10.1164/ajrccm.166.1.at1102
20. Duarte AA, Pereira CA, Rodrigues SC. Validation of new brazilian predicted values for forced spirometry in caucasians and comparison with predicted values obtained using other reference equations. J Bras Pneumol. 2007;33(5):527-35. https://doi.org/10.1590/S1806-37132007000500007
21. Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17(1):45-56. https://doi.org/10.1016/0304-3959(83)90126-4
22. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175-91. https://doi.org/10.3758/BF03193146
23. Sullivan GM, Feinn R. Using Effect Size-or Why the P Value Is Not Enough. J Grad Med Educ. 2012;4(3):279-82. https://doi.org/10.4300/JGME-D-12-00156.1
24. De Troyer A, Estenne M. Functional anatomy of the respiratory muscles. Clin Chest Med. 1988;9(2):175-93.
25. Sieck GC. Diaphragm muscle: structural and functional organization. Clin Chest Med. 1988;9(2):195-210.
26. De Troyer A, Boriek AM. Mechanics of the respiratory muscles. Compr Physiol. 2011;1(3):1273-300. https://doi.org/10.1002/cphy.c100009
27. De Troyer A, Sampson M, Sigrist S, Kelly S. How the abdominal muscles act on the rib cage. J Appl Physiol Respir Environ Exerc Physiol. 1983;54(2):465-9. https://doi.org/10.1152/jappl.1983.54.2.465
28. Luckianow GM, Ellis M, Governale D, Kaplan LJ. Abdominal compartment syndrome: risk factors, diagnosis, and current therapy. Crit Care Res Pract. 2012;2012:908169. https://doi.org/10.1155/2012/908169
29. Sánchez-Miralles A, Castellanos G, Badenes R, Conejero R. Abdominal compartment syndrome and acute intestinal distress syndrome [Article in Spanish]. Med Intensiva. 2013;37(2):99-109. https://doi.org/10.1016/j.medin.2011.11.019
30. Talisman R, Kaplan B, Haik J, Aronov S, Shraga A, Orenstein A. Measuring alterations in intra-abdominal pressure during abdominoplasty as a predictive value for possible postoperative complications. Aesthetic Plast Surg. 2002;26(3):189-92. https://doi.org/10.1007/s00266-001-1469-5
31. Perin LF, Saad R Jr, Stirbulov R, Helene A Jr. Spirometric evaluation in individuals undergoing abdominoplasty. J Plast Reconstr Aesthet Surg. 2008;61(11):1392-4. https://doi.org/10.1016/j.bjps.2008.02.028
32. Rodrigues MA, Nahas FX, Gomes HC, Ferreira LM. Ventilatory function and intra-abdominal pressure in patients who underwent abdominoplasty with plication of the external oblique aponeurosis. Aesthetic Plast Surg. 2013;37(5):993-9. https://doi.org/10.1007/s00266-013-0158-5
33. Nozawa E, Kobayashi E, Matsumoto ME, Feltrim MIZ, Carmona MJC, Auler Júnior JOC. Identificação dos fatores de risco que influenciam no desmame de ventilação mecânica em pacientes traqueostomizados após cirurgia cardíaca. Arq Bras Cardiol. 2003;80(3):301-5.
34. Rodrigues AJ, Évora PRB, Vilela V, Vicente W. Postoperative respiratory complications [Article in Portuguese]. Medicina (Ribeirao Preto). 2008;41(4):469-76. https://doi.org/10.11606/issn.2176-7262.v41i4p469-476









