ABSTRACT
Objective: Clinical trials have shown that nintedanib 150 mg twice daily (bid) reduces disease progression in patients with idiopathic pulmonary fibrosis (IPF), with an adverse event profile that is manageable for most patients. Prior to the approval of nintedanib as a treatment for IPF in Brazil, an expanded access program (EAP) was initiated to provide early access to treatment and to evaluate the safety and tolerability of nintedanib in this patient population. Methods: Patients with a diagnosis of IPF within the previous five years, forced vital capacity (FVC) ≥ 50% predicted and diffusing capacity of the lungs for carbon monoxide (DLco) 30% to 79% predicted were eligible to participate in the EAP. Patients received nintedanib 150 mg bid open-label. Safety assessments included adverse events leading to permanent discontinuation of nintedanib and serious adverse events. Results: The EAP involved 57 patients at eight centers. Most patients were male (77.2%) and white (87.7%). At baseline, mean (SD) age was 70.7 (7.5) years and FVC was 70.7 (12.5) % predicted. Mean (SD) exposure to nintedanib was 14.4 (6.2) months; maximum exposure was 22.0 months. The most frequently reported adverse events considered by the investigator to be related to nintedanib treatment were diarrhea (45 patients, 78.9%) and nausea (25 patients, 43.9%). Adverse events led to permanent discontinuation of nintedanib in 16 patients (28.1%). Sixteen patients (28.1%) had a serious adverse event. Conclusion: In the Brazilian EAP, nintedanib had an acceptable safety and tolerability profile in patients with IPF, consistent with data from clinical trials.
Keywords:
Drug tolerance; Expanded access program; Interstitial lung disease; Tyrosine kinase inhibitor.
RESUMO
Objetivo: Ensaios clínicos mostraram que 150 mg de Nintedanibe duas vezes ao dia reduzem a progressão da doença em pacientes com Fibrose Pulmonar Idiopática (FPI), com um perfil de efeitos adversos que é controlável para a maioria dos pacientes. Antes da aprovação do Ninte-danibe como tratamento para a FPI no Brasil, um Programa de Acesso Expandido (PEA) foi inicia-do para fornecer acesso precoce ao tratamento e avaliar a segurança e a tolerância do Ninteda-nibe para este grupo de pacientes. Métodos: Foram elegíveis para participar da PEA pacientes com diagnóstico de FPI nos últimos 5 anos, com capacidade vital forçada (CVF) ≥ 50% do previsto e capacidade de difusão dos pulmões para monóxido de carbono (DLco) 30%-79% do previsto. Os pacientes receberam Nintedanibe 150 mg, 2 vezes ao dia (bid). As avaliações de segurança incluí-ram eventos adversos que levaram à suspensão permanente do Nintedanibe e eventos adver-sos graves. Resultados: O PEA envolveu 57 pacientes em 8 centros. A maioria dos pacientes era do sexo masculino (77,2%) e brancos (87,7%). No início do estudo, a média de idade foi de 70,7 (7,5) anos e a CVF foi de 70,7 (12,5%) do previsto. A média de exposição ao Nintedanibe foi de 14,4 (6,2) meses; a exposição máxima foi de 22,0 meses. Os eventos adversos frequentemente relatados pelo pesquisador como relacionados ao tratamento com Nintedanibe foram diarreia (45 pacientes, 78,9%) e náusea (25 pacientes, 43,9%). Os eventos adversos levaram à suspensão permanente do Nintedanibe em 16 pacientes (28,1%) que passaram por um evento adverso grave. Conclusões: No PEA brasileiro, o Nintedanibe apresentou um perfil aceitável de segurança e tolerância em pacientes com FPI, condizendo com dados de ensaios clínicos.
Palavras-chave:
Fibrose pulmonar idiopática; Programa de acesso expandido; Doença pulmonar intersticial; Inibidor de tirosina quinase.
INTRODUCTIONIdiopathic pulmonary fibrosis (IPF) is a progressive fibrosing interstitial lung disease characterized by decline in lung function, worsening dyspnea and impaired quality of life.(1) IPF typically presents in the sixth or seventh decade of life in former smokers and is more common in men than in women.(1) IPF has a variable clinical course but a poor prognosis. Data from the US prior to the availability of approved therapies for IPF suggest that median post-diagnosis survival in patients with IPF was 3 to 5 years.(2,3) Analyses of the Brazilian National Ministry of Health Mortality Database suggest that in Brazil, mortality due to IPF rose from 0.24 per 100,000 in 1979 to 1.10 per 100,000 in 2014.(4) This increase was likely due to improved diagnosis and reporting of IPF, as well as ageing of the population. IPF likely remains significantly underdiagnosed in Brazil due to low awareness of the disease, the challenges of making the diagnosis and the small number of specialized centers.
Nintedanib is an intracellular inhibitor of tyrosine kinases involved in the pathogenesis of IPF, including the platelet derived growth factor receptor, fibroblast growth factor receptor, and vascular endothelial growth factor receptor.(5) The efficacy and safety of the 52-weeks treatment with nintedanib 150 mg twice daily (bid) in patients with IPF were assessed in the Phase II TOMORROW trial(6) and in the two Phase III INPULSIS trials.(7) These trials showed that nintedanib reduced disease progression by reducing the rate of decline in forced vital capacity (FVC). An analysis of pooled data from the INPULSIS trials suggested that nintedanib also reduced the risk of acute exacerbations.(8) The safety and tolerability profile of nintedanib was characterized predominantly by gastrointestinal adverse events, particularly diarrhea.(9,10)
Nintedanib has been approved for the treatment of IPF in many countries, including Brazil and other countries in Latin America, as well as the US, Europe and several countries in Asia. In the latest international treatment guidelines for IPF, nintedanib received a conditional recommendation for use. This indicates that it would be an appropriate choice for the majority of patients, while acknowledging that different choices will be appropriate for different patients depending on individual values and preferences.(11)
Prior to the approval of nintedanib in Brazil in February 2016, an expanded access program (EAP) was initiated to provide early access to treatment and further information on the safety and tolerability of nintedanib in patients with IPF. Here, we report the data on the safety and tolerability of nintedanib collected in this EAP.
METHODSDesignAn EAP providing open-label treatment with nintedanib was initiated at eight medical centers in Brazil in February 2015. To be eligible to participate, patients were required to be ≥ 40 years of age, with a diagnosis of IPF based on ATS/ERS/JRS/ALAT 2011 guidelines(1) within the previous 5 years, a diffusing capacity of the lungs for carbon monoxide (DLco) of 30% to 79% predicted and FVC ≥ 50% predicted. Exclusion criteria included alanine aminotransferase (ALT), aspartate aminotransferase (AST), or bilirubin levels > 1.5 times the upper limit of normal (ULN); myocardial infarction within 6 months of screening; unstable angina within 1 month of screening; bleeding risk (e.g., requirement for fibrinolysis, full-dose anticoagulation, or high-dose antiplatelet therapy); permanent discontinuation of nintedanib due to drug-related adverse events within a clinical trial; and current or planned treatment with pirfenidone, azathioprine, cyclophosphamide, cyclosporine, or prednisone at a dose of > 15 mg/day or > 30 mg every 2 days or equivalent dose of other oral corticosteroids.
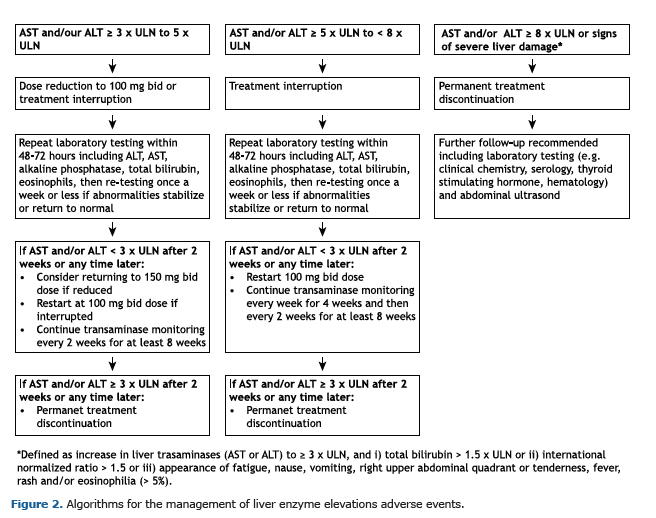
Following a 4-week screening period, patients received nintedanib 150 mg bid within the EAP until nintedanib became commercially available or until permanent treatment discontinuation. A follow-up visit took place 28 days after treatment discontinuation. Treatment interruptions for up to 12 weeks and dose reductions to 100 mg bid were allowed to manage adverse events. When dose was reduced, the dose of nintedanib could be increased back to 150 mg bid following resolution of the adverse event. The investigators were provided with recommendations for the management of diarrhea and liver enzyme elevations (Figures 1 and 2). Investigators were requested to report concomitant medications used to treat IPF or manage diarrhea on a case report form. Concomitant therapies were defined as therapies received at baseline or started between the first and last intake of nintedanib. Concomitant therapies were coded according to the WHO Drug Dictionary(12) (version 17 March).

As this was an EAP, ethics committee approval of the protocol was not mandatory. However, Independent Ethics Committees of the participating centers were sent the patient information leaflet, the informed consent form and other documents for review. The program was conducted in accordance with the principles of the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice guideline, and applicable regulatory requirements and standard operating procedures. All patients provided written informed consent before entering the program. The program was registered on www.clinicaltrials.gov (NCT02230982).
The first patient was enrolled on 23 March 2015. The last patient was screened on 3 November 2015. Nintedanib became commercially available for the treatment of IPF in Brazil on 16 February 2016. Between January 2017 and March 2017, all patients taking nintedanib in the EAP transitioned to commercially available nintedanib at the same site and with the same investigator.
OutcomesSafety was assessed in patients who received ≥ 1 dose of nintedanib. It consisted of the recording of adverse events meeting the following criteria: serious adverse events; adverse events of special interest (i.e., adverse events of liver injury [defined as AST or ALT ≥ 3 × ULN and total bilirubin ≥ 2 × ULN] or related to gastrointestinal perforation); adverse events leading to nintedanib interruption, discontinuation, or dose reduction; non-serious adverse events considered by the investigator to be related to administration of nintedanib; worsening of the underlying disease or other pre-existing conditions; changes in the results of any procedures, e.g. vital signs, physical examination, laboratory tests, that were judged clinically relevant by the investigator. Serious adverse events were defined as fatal or life-threatening adverse events, which required or prolonged hospitalization, were associated with a congenital anomaly, or resulted in a disability.
Adverse events were recorded at visits conducted at screening; at weeks 4, 8, 12, 24; every 12 weeks thereafter until the end of treatment; and at the follow-up visit 28 days after the end of treatment. They could also be recorded at any other time if the investigator became aware of them. Adverse events were coded according to preferred terms in the Medical Dictionary for Regulatory Activities (MedDRA) version 20.0. The investigator categorized the adverse events as mild (awareness of signs or symptoms which were easily tolerated), moderate (enough discomfort to cause interference with usual activity) or severe (incapacitating or causing inability to work or to perform usual activities). Safety data are presented descriptively.
RESULTSPatientsA total of 57 patients were treated with nintedanib in this EAP. The majority of them were male (77.2%), white (87.7%) and current or former smokers (64.9%); 22.8% of patients had undergone a surgical lung biopsy (Table 1). At baseline, mean (SD) age was 70.7 (7.5) years, FVC was 70.7 (12.5) % predicted and DLco was 48.7 (13.4) % predicted. The most frequent comorbid conditions at baseline were hypertension (47.4%), dyslipidemia (21.1%), gastroesophageal reflux disease (21.1%) and diabetes mellitus (17.5%) (Table 1).
 Concomitant therapies
Concomitant therapiesConcomitant therapies are shown in Table 2. Anti diarrheal therapies were the most commonly used, received by 36 patients (63.2%). Six patients (10.5%) received N-acetylcysteine and 13 patients (22.8%) received systemic corticosteroids.
 Exposure
ExposureMean (SD) exposure to nintedanib was 14.4 (6.2) months. Maximum exposure was 22.0 months. In total, 24 patients (42.1%) had ≥ 1 treatment interruption and 21 patients (36.8%) had ≥ 1 dose reduction to 100 mg bid. Most patients (70.2%) received nintedanib 150 mg bid as their last dose. Thirty-seven patients (64.9%) were still receiving nintedanib at the end of the program while 20 patients (35.1%) had permanently discontinued nintedanib. The most frequent reason for permanent discontinuation of nintedanib was adverse events (16 of 20 patients).
Safety and tolerabilityAlmost all patients (n = 55; 96.5%) had ≥ 1 adverse event that met the reporting criteria. The most frequently reported adverse events are presented in Table 3. Diarrhea, reported in 45 patients (78.9%), was the most common adverse event. Among patients with diarrhea, the intensity of the worst event was mild in 22 patients (48.9%), moderate in 13 patients (28.9%) and severe in 10 patients (22.2%). Nausea and vomiting were reported in 25 (43.9%) and 9 (15.8%) patients, respectively. Almost all nausea and vomiting adverse events were mild or moderate in intensity (Table 4). The majority of patients with adverse events of diarrhea (66.7%), nausea (80%) and vomiting (66.7%) continued nintedanib without dose reduction or treatment interruption.

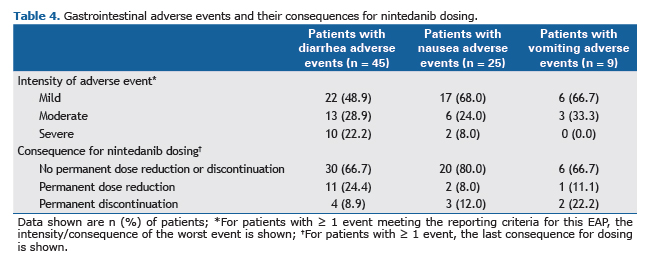
Hepatic enzymes increase was reported in 1 patient. Drug-induced liver injury was reported as a serious adverse event in 1 patient. No cases of ALT and/or AST ≥ 3 × ULN and total bilirubin ≥ 2 × ULN, or adverse events related to gastrointestinal perforation were reported.
Adverse events led to permanent discontinuation of nintedanib in 16 patients (28.1%). The adverse event that most frequently led to permanent discontinuation of nintedanib was diarrhea (4 patients; 7.0%) (Table 3). Sixteen patients (28.1%) had a serious adverse event. No type of serious adverse event (based on MedDRA preferred terms) was reported in > 1 patient, except for progression of IPF (3 patients), pneumonia (2 patients) and urinary tract infection (2 patients). Three patients (5.3%) had adverse events that led to death: pneumonia (1 patient), pneumonia and progression of IPF (1 patient), and dyspnea (1 patient). None of the fatal adverse events was considered by the investigator to be related to nintedanib.
DISCUSSIONIn this Brazilian EAP for nintedanib in patients with IPF, nintedanib 150 mg bid had acceptable safety and tolerability profile, consistent with data from clinical trials.(6,7,10) Gastrointestinal adverse events, particularly diarrhea, were the most frequently reported adverse events. Diarrhea is an adverse event commonly associated with inhibitors of tyrosine kinases, but the mechanism/s by which it occurs remains unclear.(13) Most patients who had diarrhea in the Brazilian EAP had events of mild or moderate intensity, and the majority continued nintedanib without a dose reduction or treatment interruption; however, almost two-thirds of patients received anti-diarrheal therapy. It is recommended that patients who experience diarrhea during nintedanib treatment should maintain adequate hydration and take anti-diarrheal therapy (e.g. loperamide) as soon as symptoms occur.(14,15)
Treatment with nintedanib may lead to elevations in liver enzymes and cases of drug-induced liver injury have been observed.(14,15) In the Brazilian EAP, drug-induced liver injury was reported as a serious adverse event in 1 patient. No cases of ALT and/or AST ≥ 3 × ULN and total bilirubin ≥ 2 × ULN were reported. It is recommended that liver function tests are conducted prior to initiation of nintedanib, at regular intervals during the first 3 months of treatment, and periodically thereafter.(14,15) Dose reductions or treatment interruptions may be necessary to manage elevations in liver enzymes.
In addition to this EAP in Brazil, data on the safety and tolerability of nintedanib have been collected through several other compassionate use/early access programs and through post-marketing surveillance. Consistent with our findings, data from these studies suggest that nintedanib has a similar safety and tolerability profile in clinical practice as was observed in clinical trials.(16-23) In post-marketing surveillance data from 6758 patients treated with nintedanib in the US in the year following the launch of nintedanib as a treatment for IPF, diarrhea, nausea and vomiting were the most frequently reported adverse events.(18) In an observational study of 94 patients with IPF in Greece, diarrhea was reported in 55% of patients treated with nintedanib over a follow-up period of 12 months, and 12% of patients discontinued nintedanib due to diarrhea.(20)
In the Brazilian EAP, 11% and 23% of patients treated with nintedanib received concomitant treatment with N-acetylcysteine and systemic corticosteroids, respectively. In a recent survey of 455 physicians from Latin America, 29% and 48% prescribed N-acetylcysteine and corticosteroids, respectively, for the treatment of IPF.(24) These findings suggest that use of these low-cost therapies remains high in Latin America despite the lack of evidence supporting their efficacy as treatments for IPF.(11,25,26)
In conclusion, in an EAP for patients with IPF in Brazil, nintedanib 150 mg bid had an acceptable safety and tolerability profile, consistent with that observed in clinical trials.
ACKNOWLEDGMENTSThis expanded access program for nintedanib was funded by Boehringer Ingelheim. Medical writing assistance, supported financially by Boehringer Ingelheim, was provided by Tove Anderson and Julie Fleming of FleishmanHillard Fishburn during the preparation of this article. The authors were fully responsible for all content and editorial decisions, were involved at all stages of manuscript development, and have approved the final version of the manuscript, which reflects the authors' interpretation and conclusions.
REFERENCES1. Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788-824. http://dx.doi.org/10.1164/rccm.2009-040GL. PMid:21471066.
2. Fernández Pérez ER, Daniels CE, St. Sauver J, Hartman TE, Bartholmai BJ, Yi ES, et al. Incidence, prevalence, and clinical course of idiopathic pulmonary fibrosis: a population-based study. Chest. 2010;137(1):129- 37. http://dx.doi.org/10.1378/chest.09-1002. PMid:19749005.
3. Raghu G, Chen SY, Yeh WS, Maroni B, Li Q, Lee YC, et al. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001-11. Lancet Respir Med. 2014;2(7):566-72. http://dx.doi.org/10.1016/S2213-2600(14)70101-8. PMid:24875841.
4. Algranti E, Saito CA, Silva DRME, Carneiro APS, Bussacos MA. Mortality from idiopathic pulmonary fibrosis: a temporal trend analysis in Brazil, 1979-2014. J Bras Pneumol. 2017;43(6):445-50. http://dx.doi.org/10.1590/s1806-37562017000000035. PMid:29340493.
5. Wollin L, Wex E, Pautsch A, Schnapp G, Hostettler KE, Stowasser S, et al. Mode of action of Nintedanibe in the treatment of idiopathic pulmonary fibrosis. Eur Respir J. 2015;45(5):1434-45. http://dx.doi.org/10.1183/09031936.00174914. PMid:25745043.
6. Richeldi L, Costabel U, Selman M, Kim DS, Hansell DM, Nicholson AG, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365(12):1079-87. http://dx.doi.org/10.1056/NEJMoa1103690. PMid:21992121.
7. Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of Nintedanibe in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071-82. http://dx.doi.org/10.1056/NEJMoa1402584. PMid:24836310.
8. Collard HR, Richeldi L, Kim DS, Taniguchi H, Tschoepe I, Luisetti M, et al. Acute exacerbations in the INPULSIS trials of Nintedanibe in idiopathic pulmonary fibrosis. Eur Respir J. 2017;49(5):1601339. http://dx.doi.org/10.1183/13993003.01339-2016. PMid:28526798.
9. Corte T, Bonella F, Crestani B, Demedts MG, Richeldi L, Coeck C, et al. Safety, tolerability and appropriate use of Nintedanibe in idiopathic pulmonary fibrosis. Respir Res. 2015;16(1):116. http://dx.doi.org/10.1186/s12931-015-0276-5. PMid:26400368.
10. Richeldi L, Cottin V, du Bois RM, Selman M, Kimura T, Bailes Z, et al. Nintedanibe in patients with idiopathic pulmonary fibrosis: combined evidence from the TOMORROW and INPULSIS trials. Respir Med. 2016;113:74-9. http://dx.doi.org/10.1016/j.rmed.2016.02.001. PMid:26915984.
11. Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. an update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192(2):e3-19. http://dx.doi.org/10.1164/rccm.201506-1063ST. PMid:26177183.
12. WHO: World Health Organization. [cited 2018 June 29]. Available from: http://umd.emro.who.int/WHODictionary
13. Bowen JM. Mechanisms of TKI-induced diarrhea in cancer patients. Curr Opin Support Palliat Care. 2013;7(2):162-67. http://dx.doi.org/10.1097/SPC.0b013e32835ec861. PMid:23399616.
14. Boehringer Ingelheim Pharmaceuticals, Inc. OFEV (Nintedanibe) US prescribing information [Internet]. 2018 [cited 2018 June 29]. Available from: https://www.ofev.com/
15. Boehringer Ingelheim. OFEV (Nintedanibe) EU Summary of Product Characteristics [Internet]. 2018 [cited 2018 June 29]. Available from: http://www.ema.europa.eu/ema/
16. Bonella F, Kreuter M, Hagmeyer L, Neurohr C, Keller C, Kohlhaeufl MJ, et al. Insights from the German compassionate use program of Nintedanibe for the treatment of idiopathic pulmonary fibrosis. Respiration. 2016;92(2):98-106. http://dx.doi.org/10.1159/000448288. PMid:27544537.
17. Galli JA, Pandya A, Vega-Olivo M, Dass C, Zhao H, Criner GJ. Pirfenidone and nitedanib for pulmonary fibrosis in clinical practice: tolerability and adverse drug reactions. Respirology. 2017;22(6):1171-8. http://dx.doi.org/10.1111/resp.13024. PMid:28317233.
18. Noth I, Oelberg D, Kaul M, Conoscenti CS, Raghu G. Safety and tolerability of nintedanib in patients with idiopathic pulmonary fibrosis in the USA. Eur Respir J. 2018;52(1):1702106. http://dx.doi.org/10.1183/13993003.02106-2017. PMid:29794129.
19. Brunnemer E, Wälscher J, Tenenbaum S, Hausmanns J, Schulze K, Seiter M, et al. Real-world experience with Nintedanibe in patients with idiopathic pulmonary fibrosis. Respiration. 2018;95(5):301-9. http://dx.doi.org/10.1159/000485933. PMid:29490307.
20. Tzouvelekis A, Karampitsakos T, Kontou M, Granitsas A, Malliou I, Anagnostopoulos A, et al. Safety and efficacy of Nintedanibe in idiopathic pulmonary fibrosis: a real-life observational study. Pulm Pharmacol Ther. 2018;49:61-6. http://dx.doi.org/10.1016/j.pupt.2018.01.006. PMid:29366978.
21. Bargagli E, Piccioli C, Rosi E, Torricelli E, Turi L, Piccioli E, et al. Pirfenidone and Nintedanibe in idiopathic pulmonary fibrosis: real-life experience in an Italian referral centre. Pulmonology. 2019;25(3): 149-53. http://dx.doi.org/10.1016/j.pulmoe.2018.06.003.
22. Yoon HY, Park S, Kim DS, Song JW. Efficacy and safety of Nintedanibe in advanced idiopathic pulmonary fibrosis. Respir Res. 2018;19(1):203. http://dx.doi.org/10.1186/s12931-018-0907-8. PMid:30340638.
23. Fletcher SV, Jones MG, Renzoni EA, Parfrey H, Hoyles RK, Spinks K, et al. Safety and tolerability of Nintedanibe for the treatment of idiopathic pulmonary fibrosis in routine UK clinical practice. ERJ Open Res. 2018;4(4):00049-02018. http://dx.doi.org/10.1183/23120541.00049-2018. PMid:30364342.
24. Cherrez-Ojeda I, Cottin V, Calderón JC, Delgado C, Calero E, Simanca-Racines D, et al. Management and attitudes about IPF (idiopathic pulmonary fibrosis) among physicians from Latin America. BMC Pulm Med. 2018;18(1):5. http://dx.doi.org/10.1186/s12890-017-0569-1. PMid:29321018.
25. Martinez FJ, Andrade JA, Anstrom KJ, King TE Jr, Raghu G. Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2093-101. http://dx.doi.org/10.1056/NEJMoa1401739. PMid:24836309.
26. Richeldi L, Davies HR, Ferrara G, Franco F. Corticosteroids for idiopathic pulmonary fibrosis. Cochrane Database Syst Rev. 2003;(3):CD002880. PMid:12917934.







