ABSTRACT
Objective: To investigate the efficacy of upper limb exercise training (ULExT) in improving the performance of activities of daily living (ADL) that involve the upper limbs (UL) in patients with COPD. Methods: The Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were used in this systematic review. PubMed and EBSCOhost databases were searched to identify randomized controlled trials involving adults with COPD who underwent ULExT, compared with those who underwent other types of exercise or no exercise, in order to assess the performance of ADL that involve the UL. The methodological quality of the selected studies was assessed using the Physiotherapy Evidence Database scale. Results: Five studies, with a total sample of 173 subjects, met the inclusion criteria. The results of the selected studies showed that ULExT is safe and can significantly improve the performance of ADL that involve the UL in patients with COPD. However, there were inconsistencies in the results, especially regarding the perception of symptoms during ADL. The small number of studies included and their methodological quality do not allow for firm conclusions. Conclusions: The findings of this review revealed that ULExT is a safe therapeutic approach and can improve the performance of ADL that involve the UL in patients with COPD, but the results are unclear. Further investigation through well-designed randomized trials is warranted to determine the effectiveness of ULExT in improving the performance of ADL that involve the UL in patients with COPD.
Keywords:
Upper extremity; Pulmonary disease, chronic obstructive; Activities of daily living, Exercise therapy.
RESUMO
Objectivo: Investigar a eficácia do treinamento de membros superiores (MMSS) na melhora na execução de atividades da vida diária (AVD) que envolvem os MMSS em pacientes com DPOC. Métodos: Nesta revisão sistemática foram utilizadas as diretrizes do Preferred Reporting Items for Systematic Reviews and Meta-Analyses. Os bancos de dados PubMed e EBSCOhost foram pesquisados para identificar ensaios controlados randomizados envolvendo adultos com DPOC submetidos a treinamento de MMSS, comparados àqueles submetidos a outros tipos de exercício ou a nenhum exercício, a fim de avaliar a execução de AVD que envolvem os MMSS. A qualidade metodológica dos estudos selecionados foi avaliada por meio da escala do Physiotherapy Evidence Database. Resultados: Cinco estudos, com uma amostra total de 173 indivíduos, preencheram os critérios de inclusão. Os resultados dos estudos selecionados mostraram que o treinamento de MMSS é seguro e pode melhorar significativamente a execução de AVD que envolvem os MMSS em pacientes com DPOC. No entanto, houve inconsistências nos resultados, especialmente em relação à percepção de sintomas durante as AVD. O pequeno número de estudos incluídos e a qualidade metodológica desses estudos não permitem conclusões firmes. Conclusões: Os achados desta revisão revelaram que o treinamento de MMSS é uma abordagem terapêutica segura e pode melhorar a execução de AVD que envolvem os MMSS em pacientes com DPOC, mas os resultados não são claros. São necessárias mais investigações, por meio de ensaios aleatorizados bem desenhados, para determinar a eficácia do treinamento de MMSS na melhora na execução de AVD que envolvem os MMSS em pacientes com DPOC.
Palavras-chave:
: Extremidade superior; Doença pulmonar obstrutiva crônica; Atividades cotidianas; Terapia por exercício.
INTRODUCTIONCOPD is a chronic respiratory disease that has high rates of mortality and morbidity.(1) Dyspnea is one of the major symptoms of patients with COPD, limiting their physical activity and affecting their ability to perform activities of daily living (ADL).(2) More specifically, activities that require elevation of upper limbs (UL) over the shoulder height may disrupt normal breathing and cause hyperinflation,(3) which is associated with the occurrence of dyspnea.(2) In such patients, ADL tasks using the UL have been shown to increase metabolic and ventilatory costs, as assessed by the oxygen uptake/maximal oxygen uptake ratio (expressed as a percentage); the minute ventilation/maximal voluntary ventilation ratio (expressed as a percentage); and oxygen pulse. These increments could explain tiredness and be associated with increased perception of dyspnea, which leads to limitation of ADL.(4) In addition, some pathological muscle changes (such as weakness and loss of mass) that might occur in patients with COPD(5) also contribute to the increase in dyspnea and early fatigue.(6,7) For all of these reasons, patients with COPD suffer from shortness of breath during ADL when they use the UL,(8-10) such as when cooking or driving, and they might try to avoid such ADL in order to avoid dyspnea.
Pulmonary rehabilitation programs, including exercise training, have shown to be able to reduce dyspnea and fatigue in patients with COPD and improve their exercise capacity and quality of life.(11) However, recent guidelines for pulmonary rehabilitation are unclear about whether UL exercises should be performed as part of exercise training.(11,12)
Improving the performance of ADL should be one of the most important goals of rehabilitation, because it could affect the ability of patients for self-care. For this reason, the present study undertook a systematic review of randomized controlled trials to investigate whether UL exercise training (ULExT) can improve the performance of ADL in patients with COPD and whether ULExT is safe for such patients.
METHODSThe article selection process was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines(13,14) in the present systematic review. We searched the following electronic databases (from inception to May of 2019): PubMed and EBSCOhost (CINAHL Plus and SPORTDiscus). The search was limited to literature written in English. The selected keywords were related to COPD ("COPD"; "chronic obstructive pulmonary disease"; "chronic obstructive lung disease"; "chronic obstructive airway disease"; "emphysema"; "chronic airflow limitation"; and "chronic airway obstruction"), UL ("arm"; "upper extremity"; and "upper limb"), exercise ("exercise therapy"; "exercise"; "respiratory rehabilitation"; "pulmonary rehabilitation"; "physical exercise"; "physiotherapy"; "physical therapy"; "training"; "exercise capacity"; "exercise test"; and "exercise endurance"), and symptoms ("dyspnea" and "fatigue"). Articles retrieved were perused for other relevant references.
The search yield was initially screened by two independent reviewers in order to remove duplicates and to assess titles and abstracts of potentially relevant articles. Full-text articles were retrieved when the title or abstract provided insufficient information to determine article inclusion. In cases of disagreement, a consensus was reached by discussion, and, if necessary, a third reviewer made the decision regarding article inclusion/exclusion.
In order to be included in our systematic review, the articles had to meet criteria that were developed following the PICOS-an acronym for Population of interest, Intervention, Comparison, Outcome, and Study design-approach. In the present review, the criteria were as follows: P = men and women over 18 years of age, diagnosed with COPD (regardless of severity); I = any form of ULExT program; C = three types of comparison (ULExT vs. no ULExT; ULExT and lower limb exercise training vs. lower limb exercise training only; and comparison between two types of ULExT); O = performance of ADL; and S = randomized controlled trials.
It is worth mentioning that the primary outcome of the present review was the performance of ADL that involve the UL. The ability to perform various ADL is influenced by symptoms of dyspnea and fatigue in patients with COPD. For this reason, the perception of these symptoms was a secondary outcome in this review. The safety of ULExT was also a secondary outcome measure, the studies included being screened for reported adverse events related to the exercise program.
A customized Excel workbook was used for data extraction based on a recent systematic review about ULExT in COPD,(15) including methods (study design, total duration of study, details of any run-in period, number of study centers and their location, study setting, withdrawals, and date of study); participants (number, mean age, gender, severity of COPD, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, and exclusion criteria); interventions (intervention and comparison groups); outcomes (primary and secondary outcomes and reported time points); results of outcome measures; and notes (funding and conflicts of interest).
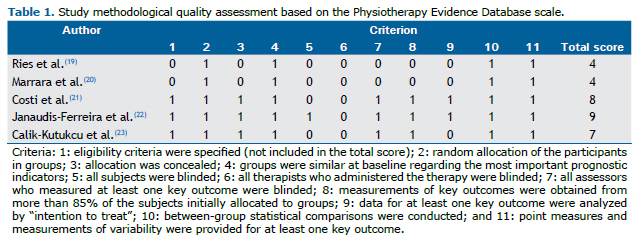
The quality and the risk of bias of the studies were assessed based on some methodological and statistical criteria (e.g., randomization, blinding, data comparison before and after the intervention and between groups, etc.). The methodological quality of each study was assessed independently by two investigators using the Physiotherapy Evidence Database (PEDro) scale, which is based on the criteria of the Delphi List(16) and is considered to be valid and reliable.(17,18) A study with a PEDro scale score ≥ 7 is considered to have high methodological quality, whereas those with a score between 4 and 6 and those with a score ≤ 3, respectively, are considered to have intermediate and poor methodological quality.
RESULTSDuring the initial search in the electronic databases, 201 articles were found. Only five studies met all the aforementioned inclusion criteria and had the data required for investigating the effectiveness of ULExT in patients with COPD (Figure 1).(19-23)
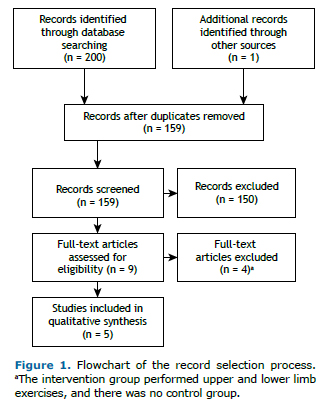
In accordance with the PEDro scale, the methodological quality of the studies was moderate (two studies) to high (three studies), with scores ranging from 4 to 9 (Table 1). All studies referred to the comparison of the outcome measures between the groups. In two of the trials, there was no form of blinding (either for participants or for researchers or examiners).
The total sample size of each study ranged from 22 to 50 patients, with the number of participants per group ranging from 6 to 25. The five studies together involved 173 patients. The majority of the patients in the studies were men. In one of the studies, all of the participants were male.(20) The overall mean age of the participants was 66 years. In one of the studies, sex and age of the patients were unavailable.(19) The characteristics of the participants are presented in Table 2.
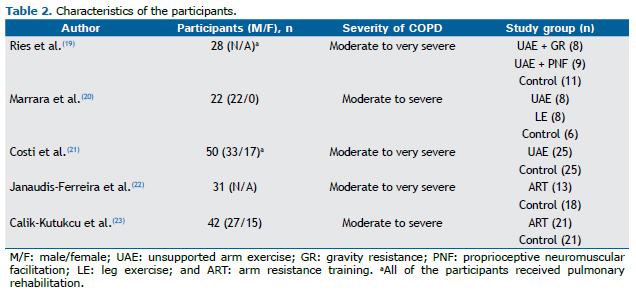
Overall, the patients had moderate to very severe COPD as determined by the Global Initiative for Chronic Obstructive Lung Disease criteria.(24) In all studies, having stable COPD was an inclusion criterion, whereas the presence of an exacerbation three weeks to two months prior to the study was considered an exclusion criterion in most of the studies.(20-23)
Of the five studies, four reported that the diagnosis of COPD was based on spirometry results, and one did not specify how COPD was diagnosed.(19) In addition, two of the studies also included the presence of dyspnea or fatigue during ADL as an inclusion criterion.(22,23)
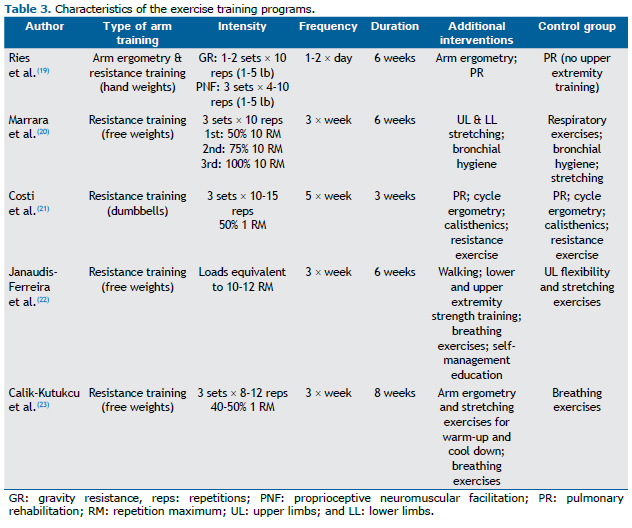
In all of the studies, the samples were divided into groups, of which at least one group followed a ULExT program. Samples were divided into two groups in three studies(21-23) and into three groups in two studies. (19,20) The intervention group performed unsupported arm resistance exercise (i.e., using various weights such as dumbbells and hand weights) in all studies.(19-23) Control groups performed breathing exercises,(23) respiratory hygiene,(20) or participated in a pulmonary rehabilitation program with no ULExT.(19,21) In one of the studies, the control group performed sham UL exercises which included flexibility exercises and stretching.(22)
The total duration of the training programs ranged from three to eight weeks. In most studies, there were three training sessions per week.(20,22,23) In one study, the total duration of the training program was three weeks (five training sessions per week).(21) In another study, the first week of training consisted of one session daily, followed by two daily sessions during the following weeks.(19) Most of the training programs were supervised; however, participants were supervised "approximately weekly" in one study.(19)
Various parameters were used in order to determine exercise intensity. In strength training programs, exercise intensity was determined by the load of weights (in pounds)(19) and by the number of sets and repetitions.(20-23) In one of the studies, they also used the number of arm motions per exhalation to determine exercise intensity.(19) In all of the studies, there was a gradual increase in exercise intensity taking into consideration the ability of the patients to complete the whole program (sets and repetitions) with no interruption.(19) Exercise intensity was increased based on the absence of dyspnea, fatigue, or muscle pain in three studies.(21-23) Finally, in one of the studies, the training load was adjusted based on a ten-repetition maximum test in the middle of the program period. (20) The characteristics of the training programs are presented in Table 3.
Although attrition was reported in four of the studies,(19,20,22,23) noncompliance with the training program was mentioned only in two (1 patient each). (19,22) The reasons for noncompliance and adverse events reported in each study were investigated in order to evaluate the safety of ULExT in patients with COPD. The overall number of dropouts was 35 patients: 2 presented with an exacerbation(22,23) during the training program (which was reported to be unrelated to the training program in 1)(22); 14 dropped out for medical reasons, 13 of whom unrelated to COPD or to the training program, such as prostate cancer (in 1),(23) and 1 presented with back pain, which was probably related to the training program(19); 9 dropped out for nonmedical reasons (2 being noncompliant patients); 3 were unable to participate in the final evaluation(23); and 7 were lost to follow-up (and no further information was available).(20) In addition, 3 complained of pain or severe dyspnea (it was unclear whether or not those complaints were related to the training program; however, none of those patients discontinued the program, which was adapted to their needs).(22)
The performance of ADL that involve the UL was an outcome measure in four studies.(19-21,23) Assessment of the performance of ADL included blackboard erasing,(19-21,23) dishwashing,(19,21,23) shelving groceries or lifting weight,(19-21,23) and changing light bulbs.(21,23) The patients were asked to perform the activities within a given timeframe(20,21,23) or the time needed to complete the ADL tasks was recorded.(19) In one study, the Glittre-ADL test(25,26) was also used in order to evaluate the performance of ADL.(23) The Borg scale was used in order to determine the perception of dyspnea or fatigue before, during, and after the simulation tests. Two studies used tools specific for the assessment of UL activities, such as the Milliken ADL Scale(23) and a modified version of the Disabilities of the Arm, Shoulder and Hand Questionnaire,(22) in order to assess ADL. Finally, in one study, patients were asked to rate their perception of dyspnea during the tests using the dyspnea domain of the Chronic Respiratory Disease Questionnaire.(22) In one of the studies, there was a significant decrease in the time taken to complete the ADL test, a greater decrease being seen in the ULExT group undergoing proprioceptive neuromuscular facilitation.(19) In addition, two studies(21,23) that used an ADL test similar to that of the study above(19) showed a significant increase in the number of completed cycles in the ULExT group compared with the control group(21) or with baseline.(23) Another study evaluated the metabolic response during ADL tests and revealed that the minute ventilation/maximal voluntary ventilation ratio and the oxygen uptake/maximal oxygen uptake ratio decreased during blackboard erasing in the ULExT group.(20) One study showed significant improvement in Milliken ADL Scale scores in the ULExT group.(23) However, no significant changes were found in the Glittre-ADL test results(23) or in Disabilities of the Arm, Shoulder and Hand questionnaire scores.(22)
In three studies, the perception of dyspnea showed no significant changes during UL-related ADL simulation tests.(19,20,23) In only one study did the perception of dyspnea significantly improve in both groups.(21) In another study, there was a significant reduction in the perception of dyspnea during the Glittre-ADL test in the ULExT group.(23) Finally, the perception of dyspnea during ADL tests significantly changed in both groups in one study.(22)
Fatigue during ADL assessment showed no significant changes in two studies.(19,23) Only in one study did arm fatigue show a significant improvement during ADL simulation testing in the intervention group.(21) The results in the ULExT groups are presented in Table 4.
 DISCUSSION
DISCUSSIONThe aim of the present systematic review was to investigate the potential effectiveness of ULExT in improving the performance of ADL that involve the upper limbs in patients with COPD. Our results indicate that ULExT is a safe therapeutic approach for these patients. In addition, significant improvements in the performance of ADL that involve the UL were found in the ULExT group compared with baseline(20,23) or compared with the control group.(21) However, ULExT has shown contradictory results in symptom perception during ADL that involve the UL.
ADL, especially activities involving the UL, are compromised in patients with COPD. The results of four of the studies that examined the effect of ULExT in UL-related ADL simulation tests showed that ULExT can provide significant improvements (Table 4).(19-21,23) In one study, there was a significant reduction in the total time taken to complete the ADL tasks.(19) This finding was also seen in two other studies, in which there was a significant increase in the number of cycles completed during the ADL simulation test within a ten-minute timeframe in the ULExT group.(21,23) Finally, in another study, a significant decrease in metabolic and ventilatory demand during blackboard erasing was seen only in the ULExT group.(20)
Although significant improvements were seen in the intervention groups, there were some contradictory findings in some of these studies. In one of the studies, in which there was a significant difference in group-time interaction in the total time taken to complete the ADL tasks, there was no significant effect of group or time factors.(19) This finding can be attributed to the fact that the time taken to complete the ADL test at baseline was longer in the group undergoing proprioceptive neuromuscular facilitation than in the other groups; however, no significant differences were found.(19) In another study, there was a significant increase in the number of cycles performed during the ADL simulation test both in the intervention and control groups.(23)
Symptom perception might explain the improvements in the performance of ADL during the tests. It is well known that the occurrence of symptoms of dyspnea and fatigue might restrict UL activities.(9) One study reported that improved symptom perception correlated with improved performance of ADL.(21) However, in one study, no significant improvement in the perception of dyspnea or fatigue during ADL simulation tests was reported, although the number of cycles completed during the tests significantly increased in the intervention and control groups.(23) This phenomenon might be attributed to the respiratory exercises that both groups performed.(27) It is well known that the use of UL can cause hyperinflation,(3,10) which is associated with the occurrence of dyspnea(2) and dysfunction of intrinsic contractile properties of respiratory muscles. (28) Nevertheless, hyperinflation is not the sole cause of dyspnea. Various studies have clearly shown that dyspnea is a complex symptom and is triggered by many factors, such as emotions, cognition, context, and pathophysiology.(29) According to these findings, it seems logical that arm training and breathing exercises can improve hyperinflation and pulmonary function during UL activities without necessarily improving the perception of dyspnea. This assumption seems to be confirmed in one of the studies included in this review, in which there was a decrease in metabolic and ventilatory demand during blackboard erasing but no significant change in the perception of dyspnea in the ULExT group.(20)
In some cases, ADL were assessed by means of tests or questionnaires. In one study, the Glittre-ADL test was used, but no significant changes were found within or between the groups.(23) This test requires getting out of a chair, walking ten meters, walking up and down a ladder while carrying a weighted backpack, and moving objects in a bookcase.(25,26) The lack of significant results using this test might be because it requires the performance of activities that involve the lower limbs (such as walking); exercises involving the lower limbs were not included in the ULExT in that study.(23) However, activities such as housecleaning and doing laundry showed significant changes according to ADL questionnaire scores.(23) Nevertheless, questionnaires have the disadvantage of providing subjective answers that are not always real.
Although significant improvements were found in some of the studies included in the present review, the aforementioned findings cannot be generalized because of some quantitative and qualitative limitations of those studies. The number of studies included was small (only five), which seems quite insufficient to provide clear results. In addition, according to the PEDro scale, the methodological quality of the studies was moderate, in two studies, and high, in three studies, and there was no blinding of researchers in two studies.(19,20) In such cases, their personal beliefs in favor of or against an intervention can alter the results (information bias).(30,31)
In most of the studies included in this review, the sample size was not determined on the basis of a statistical power analysis, and, in some cases, the number of participants in each group was smaller than 11 patients (pilot studies). In addition, the majority of the samples comprised males. The severity of COPD, in accordance with the Global Initiative for Chronic Obstructive Lung Disease criteria, ranged from moderate to very severe; no patients with mild COPD were included in any of the trials.
Apart from the limitations in the methodological design, there were several differences regarding the ULExT among the selected studies, such as the frequency of ULExT sessions (from three times a week to twice daily), total duration of the program (ranging from three to eight weeks), duration of the sessions, type of exercise, and exercise intensity. In addition, the activities proposed to the control groups were different. Another limitation involves the assessment of ADL. Four studies used nonvalidated simulation tests to assess ADL.(19-21,23) Moreover, there were differences in the methods of assessment and in outcome measures to evaluate ADL, the number of ADL tasks being two in one trial,(20) three in another trial,(19) and four in two trials.(21,23) Furthermore, the outcome measure of ADL simulation tests was time, measured in seconds, in one study,(19) or the number of cycles completed in ten minutes, in two studies.(21,23) All of these limitations prevent generalization of the findings of the studies. Because of these discrepancies, a meta-analysis could not be performed.
Three previous systematic reviews investigated ULExT and the ability to perform ADL that involve the UL in patients with COPD.(15,32,33) The number of trials included in the reviews was smaller than was the number of trials in our study, ranging from one to three. The outcome measure was the performance of ADL or symptoms during ADL. One of the three reviews concluded that ULExT should not be recommended,(32) and two concluded that there was no clinical evidence to support the efficacy of ULExT in improving symptoms during ADL,(15,33) one of which reporting a general improvement in dyspnea but not during ADL.(15) The trials included in those reviews(15,32,33) were also included in the present systematic review, with the addition of two other studies. Our review demonstrated that, in four of the five studies included, the performance of ADL that involve the UL in the ULExT group improved significantly compared with baseline(19,20,23) or with the control group.(21) A decrease in the perception of dyspnea during ADL in the ULExT group was reported in three studies,(21-23) whereas a significant decrease in fatigue in the ULExT group, compared with the control group, was reported only in one study.(21)
ULExT can be considered safe for patients with COPD, because no deterioration of the disease or any other severe adverse effects, which could lead to hospitalization or death, have been reported.
A major goal of pulmonary rehabilitation, especially ULExT, is improving the performance of ADL in patients with COPD. The findings of this review revealed that ULExT is safe and can significantly improve the performance of ADL that involve the UL in such patients. However, the limitations of the selected studies and their contradictory results regarding the symptoms of dyspnea and fatigue during ADL that involve the UL bring uncertainty regarding the results. Further research through well-designed randomized controlled trials is warranted to determine the efficacy of ULExT and the proper parameters for this type of training in patients with COPD.
AUTHOR CONTRIBUTIONSAll authors have contributed to the preparation of the manuscript (study design, interpretation of findings, and drafting, writing, and revising of the manuscript) and have read and approved the final version for submission.
REFERENCES1. Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347-365. https://doi.org/10.1164/rccm.201204-0596PP
2. O'Donnell DE, Laveneziana P. Dyspnea and activity limitation in COPD: mechanical factors. COPD. 2007;4(3):225-236. https://doi.org/10.1080/15412550701480455
3. Gigliotti F, Coli C, Bianchi R, Grazzini M, Stendardi L, Castellani C, et al. Arm exercise and hyperinflation in patients with COPD: effect of arm training. Chest. 2005;128(3):1225-1232. https://doi.org/10.1378/chest.128.3.1225
4. Velloso M, Stella SG, Cendon S, Silva AC, Jardim JR. Metabolic and ventilatory parameters of four activities of daily living accomplished with arms in COPD patients. Chest. 2003;123(4):1047-1053. https://doi.org/10.1378/chest.123.4.1047
5. Maltais F, Decramer M, Casaburi R, Barreiro E, Burelle Y, Debigaré R, et al. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):e15-e62. https://doi.org/10.1164/rccm.201402-0373ST
6. Kobayashi A, Yoneda T, Yoshikawa M, Ikuno M, Takenaka H, Fukuoka A, et al. The relation of fat-free mass to maximum exercise performance in patients with chronic obstructive pulmonary disease. Lung. 2000;178(2):119-127. https://doi.org/10.1007/s004080000014
7. Gea J, Orozco-Levi M, Barreiro E, Ferrer A, Broquetas J. Structural and functional changes in the skeletal muscles of COPD patients: the "compartments" theory. Monaldi Arch Chest Dis. 2001;56(3):214-224.
8. Tangri S, Woolf CR. The breathing pattern in chronic obstructive lung disease during the performance of some common daily activities. Chest. 1973;63(1):126-127. https://doi.org/10.1378/chest.63.1.126
9. Breslin EH. Dyspnea-limited response in chronic obstructive pulmonary disease: reduced unsupported arm activities. Rehabil Nurs. 1992;17(1):12-20. https://doi.org/10.1002/j.2048-7940.1992.tb01254.x
10. Lima VP, Iamonti VC, Velloso M, Janaudis-Ferreira T. Physiological Responses to Arm Activity in Individuals With Chronic Obstructive Pulmonary Disease Compared With Healthy Controls: A SYSTEMATIC REVIEW. J Cardiopulm Rehabil Prev. 2016;36(6):402-412. https://doi.org/10.1097/HCR.0000000000000190
11. Bolton CE, Bevan-Smith EF, Blakey JD, Crowe P, Elkin SL, Garrod R, et al. British Thoracic Society guideline on pulmonary rehabilitation in adults. Thorax. 2013;68 Suppl 2:ii1-ii30. https://doi.org/10.1136/thoraxjnl-2013-203808
12. Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation [published correction appears in Am J Respir Crit Care Med. 2014 Jun 15;189(12):1570]. Am J Respir Crit Care Med. 2013;188(8):e13-e64.
13. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. https://doi.org/10.1371/journal.pmed.1000100
14. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement [published correction appears in Int J Surg. 2010;8(8):658]. Int J Surg. 2010;8(5):336-341. https://doi.org/10.1016/j.ijsu.2010.02.007
15. McKeough ZJ, Velloso M, Lima VP, Alison JA. Upper limb exercise training for COPD. Cochrane Database Syst Rev. 2016;11(11):CD011434. https://doi.org/10.1002/14651858.CD011434.pub2
16. Verhagen AP, de Vet HC, de Bie RA, Kessels AG, Boers M, Bouter LM, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51(12):1235-1241. https://doi.org/10.1016/S0895-4356(98)00131-0
17. Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83(8):713-721. https://doi.org/10.1093/ptj/83.8.713
18. de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. 2009;55(2):129-133. https://doi.org/10.1016/S0004-9514(09)70043-1
19. Ries AL, Ellis B, Hawkins RW. Upper extremity exercise training in chronic obstructive pulmonary disease. Chest. 1988;93(4):688-692. https://doi.org/10.1378/chest.93.4.688
20. Marrara KT, Marino DM, de Held PA, de Oliveira Junior AD, Jamami M, Di Lorenzo VA. Different physical therapy interventions on daily physical activities in chronic obstructive pulmonary disease. Respir Med. 2008;102(4):505-511. https://doi.org/10.1016/j.rmed.2007.12.004
21. Costi S, Crisafulli E, Degli Antoni F, Beneventi C, Fabbri LM, Clini EM. Effects of unsupported upper extremity exercise training in patients with COPD: a randomized clinical trial. Chest. 2009;136(2):387-395. https://doi.org/10.1378/chest.09-0165
22. Janaudis-Ferreira T, Hill K, Goldstein RS, Robles-Ribeiro P, Beauchamp MK, Dolmage TE, et al. Resistance arm training in patients with COPD: A Randomized Controlled Trial. Chest. 2011;139(1):151-158. https://doi.org/10.1378/chest.10-1292
23. Calik-Kutukcu E, Arikan H, Saglam M, Vardar-Yagli N, Oksuz C, Inal-Ince D, et al. Arm strength training improves activities of daily living and occupational performance in patients with COPD. Clin Respir J. 2017;11(6):820-832. https://doi.org/10.1111/crj.12422
24. Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195(5):557-582. https://doi.org/10.1164/rccm.201701-0218PP
25. Skumlien S, Hagelund T, Bjørtuft O, Ryg MS. A field test of functional status as performance of activities of daily living in COPD patients. Respir Med. 2006;100(2):316-323. https://doi.org/10.1016/j.rmed.2005.04.022
26. Dechman G, Scherer SA. Outcome Measures in Cardiopulmonary Physical Therapy: Focus on the Glittre ADL-Test for People with Chronic Obstructive Pulmonary Disease. Cardiopulm Phys Ther J. 2008;19(4):115-118. https://doi.org/10.1097/01823246-200819040-00003
27. Borge CR, Hagen KB, Mengshoel AM, Omenaas E, Moum T, Wahl AK. Effects of controlled breathing exercises and respiratory muscle training in people with chronic obstructive pulmonary disease: results from evaluating the quality of evidence in systematic reviews. BMC Pulm Med. 2014;14:184. https://doi.org/10.1186/1471-2466-14-184
28. O'Donnell DE, Webb KA, Neder JA. Lung hyperinflation in COPD: applying physiology to clinical practice. COPD Research Pract. 2015;1:4. https://doi.org/10.1186/s40749-015-0008-8
29. Hayen A, Herigstad M, Pattinson KT. Understanding dyspnea as a complex individual experience. Maturitas. 2013;76(1):45-50. https://doi.org/10.1016/j.maturitas.2013.06.005
30. Karanicolas PJ, Farrokhyar F, Bhandari M. Practical tips for surgical research: blinding: who, what, when, why, how?. Can J Surg. 2010;53(5):345-348.
31. Schulz KF, Grimes DA. Blinding in randomised trials: hiding who got what. Lancet. 2002;359(9307):696-700. https://doi.org/10.1016/S0140-6736(02)07816-9
32. Costi S, Di Bari M, Pillastrini P, D'Amico R, Crisafulli E, Arletti C, et al. Short-term efficacy of upper-extremity exercise training in patients with chronic airway obstruction: a systematic review. Phys Ther. 2009;89(5):443-455. https://doi.org/10.2522/ptj.20070368
33. PAN L, GUO YZ, YAN JH, ZHANG WX, SUN J, LI BW. DOES UPPER EXTREMITY EXERCISE IMPROVE DYSPNEA IN PATIENTS WITH COPD? A META-ANALYSIS. RESPIR MED. 2012;106(11):1517-1525. HTTPS://DOI.ORG/10.1016/J.RMED.2012.08.002








