ABSTRACT
Objective: Impaired respiratory mechanics and gas exchange may contribute to sleep disturbance in patients with COPD. We aimed to assess putative associations of different domains of lung function (airflow limitation, lung volumes, and gas exchange efficiency) with polysomnography (PSG)-derived parameters of sleep quality and architecture in COPD. Methods: We retrospectively assessed data from COPD 181 patients = 40 years of age who underwent spirometry, plethysmography, and overnight PSG. Univariate and multivariate linear regression models predicted sleep efficiency (total sleep time/total recording time) and other PSG-derived parameters that reflect sleep quality. Results: The severity of COPD was widely distributed in the sample (post-bronchodilator FEV1 ranging from 25% to 128% of predicted): mild COPD (40.3%), moderate COPD (43.1%), and severe-very severe COPD (16.6%). PSG unveiled a high proportion of obstructive sleep apnea (64.1%) and significant nocturnal desaturation (mean pulse oximetry nadir = 82.2% ± 6.9%). After controlling for age, sex, BMI, apnea-hypopnea index, nocturnal desaturation, comorbidities, and psychotropic drug prescription, FEV1/FVC was associated with sleep efficiency (ß = 25.366; R2 = 14%; p < 0.001), whereas DLCO predicted sleep onset latency (ß = -0.314; R2 = 13%; p < 0.001) and rapid eye movement sleep time/total sleep time in % (ß = 0.085; R2 = 15%; p = 0.001). Conclusions: Pulmonary function variables reflecting severity of airflow and gas exchange impairment, adjusted for some potential confounders, were weakly related to PSG outcomes in COPD patients. The direct contribution of the pathophysiological hallmarks of COPD to objectively measured parameters of sleep quality seems to be less important than it was previously assumed.
Keywords:
Pulmonary disease, chronic obstructive; Respiratory function tests; Sleep; Sleep apnea, obstructive; Comorbidity.
RESUMO
Objetivo: O comprometimento da mecânica respiratória e das trocas gasosas pode contribuir para distúrbios do sono em pacientes com DPOC. Objetivamos avaliar associações putativas de diferentes domínios da função pulmonar (limitação do fluxo aéreo, volumes pulmonares e eficiência das trocas gasosas) com parâmetros da qualidade e arquitetura do sono na DPOC derivados da polissonografia (PSG). Métodos: Avaliamos retrospectivamente dados de 181 pacientes com DPOC e idade ≥ 40 anos que foram submetidos a espirometria, pletismografia e PSG de noite inteira. Modelos de regressão linear univariada e multivariada foram utilizados para avaliar a associação de variáveis de função pulmonar com a eficiência do sono (tempo total de sono/tempo total de registro) e outros parâmetros derivados da PSG que refletem a qualidade do sono. Resultados: A gravidade da DPOC foi bem distribuída na amostra (VEF1 pós-broncodilatador variando de 25% a 128% do previsto): DPOC leve (40,3%), DPOC moderada (43,1%) e DPOC grave-muito grave (16,6%). A PSG revelou uma alta frequência de apneia obstrutiva do sono (64,1%) e dessaturação noturna significativa (nadir médio da oximetria de pulso = 82,2% ± 6,9%). Após controle para idade, sexo, IMC, índice de apneia-hipopneia, dessaturação noturna, comorbidades e prescrição de psicotrópicos, a relação VEF1/CVF apresentou associação com a eficiência do sono (β = 25,366; R2 = 14%; p < 0,001), enquanto a DLCO previu a latência para o início do sono (β = −0,314; R2 = 13%; p < 0,001) e o tempo de sono rapid eye movement/tempo total de sono em % (β = 0,085; R2 = 15%; p = 0,001). Conclusões: As variáveis de função pulmonar que refletem a gravidade do comprometimento do fluxo aéreo e das trocas gasosas, ajustadas para alguns potenciais fatores de confusão, apresentaram fraca relação com os resultados da PSG nos pacientes com DPOC. A contribuição direta das características fisiopatológicas da DPOC para os parâmetros da qualidade do sono medidos objetivamente parece ser menos importante do que se supunha anteriormente.
Palavras-chave:
Doença pulmonar obstrutiva crônica; Testes de função respiratória; Sono; Apneia obstrutiva do sono; Comorbidade.
INTRODUCTION COPD can potentiate the complex effects of disturbed sleep on the respiratory system, including changes in central respiratory control, airway resistance, gas exchange, and respiratory muscle contractility.(1) In fact, patients with COPD frequently report impaired sleep,(2-4) which was ranked as the third most troublesome disturbance, after dyspnea and fatigue.(2) They also endorse the morning as the worst time of the day vis-à-vis energy levels and willingness to undertake activities of daily living.(5) Accordingly, low sleep efficiency,(6,7) disturbed sleep architecture,(3) and challenges in initiating and maintaining sleep(3,4,8) have been confirmed by overnight polysomnography (PSG) in this population of patients.
The mechanisms leading to impaired sleep in COPD are still controversial.(9,10) Altered respiratory mechanics and gas exchange abnormalities(11) may render patients more susceptible to nocturnal hypoventilation and hypoxemia. Previous studies demonstrated that airflow obstruction(7) and lung hyperinflation(7,12) were correlated with poorer sleep quality, whereas nocturnal O2 desaturation may disrupt normal sleep architecture.(13) Reduction in the neural respiratory drive to the respiratory muscles during sleep(14) may also contribute to nocturnal hypoventilation and sleep disturbances. Unfortunately, moreover, sleep quality may also be negatively affected by a plethora of factors that are common in COPD patients, such as senescence, obesity, cardiovascular/metabolic comorbidities,(15) and polypharmacy.(10) These features are even more prevalent in patients with greater lung function impairment.(15) Accordingly, we hypothesized that the effect(s) of resting pulmonary function abnormalities on impaired sleep quality(7,12,13) could be influenced by some of these features, such as obesity, nocturnal (de)oxygenation, comorbidities, psychotropic drug prescription, and/or alcohol consumption), which are frequently observed in elderly individuals with COPD.
Our objective, therefore, was to assess—after careful adjustment for the abovementioned confounders—putative associations of different domains of lung function (airflow limitation, lung volumes, and gas exchange efficiency) with PSG-derived parameters of sleep quality and architecture in COPD.
METHODS Study design and population This was a retrospective cross-sectional study. Using pre-specified criteria, data of all consecutive patients ≥ 40 years of age who were referred to the Clinical Laboratories of Queen’s University Affiliated Teaching Hospitals (Kingston General Hospital and Hotel Dieu Hospital, both located in the city of Kingston, Canada) for spirometry with post-bronchodilator assessment, whole-body plethysmography, DLCO, and overnight PSG between 2008 and 2016 were reviewed (Figure 1). These exams were requested at the discretion of the attending physicians to evaluate respiratory (lung function) and sleep-related (PSG) complaints. In the case of sequential pulmonary function measurements, the last assessment was recorded for analysis. The following data were obtained from PSG reports: age, sex, BMI, smoking status (former/current smoker vs. never smoker), main diagnosis, comorbidities, alcohol consumption (on the day of the exam), and medicine prescription.
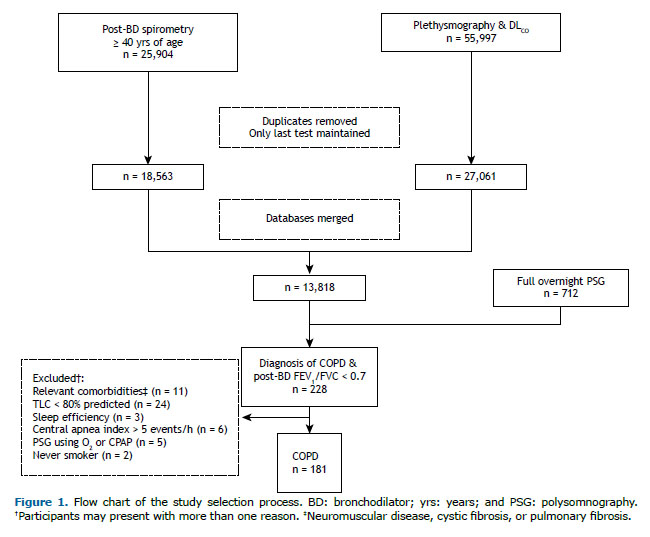
Participants were included based on informed diagnosis or suspicion of COPD by the attending physician, post-bronchodilator (albuterol, 400 µg) FEV1/FVC < 0.70, and previous or current history of smoking. Exclusion criteria included conditions that could affect sleep quality (neuromuscular disease, previous stroke with neurologic sequelae, or active cancer), chronic respiratory disease (bronchiectasis, interstitial lung disease, TLC < 80% of the predicted values), lack of sleep during PSG (total sleep time (TST)/total recording time [sleep efficiency] < 20%), central apnea index > 5 events/h, and/or use of nocturnal CPAP or oxygen supplementation.
Subjects were unnamed and identified by unique identification numbers. The study (#6020749) was approved by the Queen’s University Health Sciences and Affiliated Teaching Hospitals Research Ethics Board (FWA #00004184; IRB #00001173). Informed consent was waived by the institutional research committee given the retrospective design of the study and the guarantee of anonymity of all individual data included in the study.
Procedures Spirometry (including inspiratory capacity [IC]), body plethysmography, and DLCO measurements were performed with automated testing equipment (V6200 Autobox; SensorMedics, Yorba Linda, CA, USA) in the Pulmonary Function Laboratory at Hotel Dieu Hospital in accordance with international standards (American Thoracic Society/European Respiratory Society).
Standard PSG measurements were collected in the Sleep Laboratory at Kingston General Hospital. Continuous recordings using the Sandman Elite SD 32+ digital sleep recording system (Embla; Mallinckrodt/Nellcor Puritan Bennett [Melville] Ltd, Mansfield, MA, USA) included four electroencephalography channels (C4A1, C3A2, O2A1, and O1A2); two electrooculogram channels (ROCA1 and LOCA2); submental electromyography; bilateral anterior tibialis electromyography; electrocardiography; chest and abdominal respiratory belts; nasal pressure via nasal cannula; finger pulse oximeter (SpO2); and a vibration snore sensor. Sleep was staged, and obstructive apneas and hypopneas were defined using established criteria. (16) Apneas were defined as central if there was a lack of respiratory effort during the period of absent airflow. Daytime sleepiness was assessed using the Epworth Sleepiness Scale. A score equal to or higher than 10 points was considered as excessive daytime sleepiness. Obstructive sleep apnea (OSA) was defined as an apnea/hypopnea index (AHI) ≥ 5 events/h and accompanied by excessive daytime sleepiness or an AHI ≥ 15 events/h regardless of coexistent symptoms. (17) Sleep efficiency values < 85% were defined as abnormally low.(18,19) Pulmonary function tests and PSG were routinely performed only if the subject was clinically stable in the preceding four weeks.
Statistical analysis Statistical analysis was performed with the IBM SPSS Statistics software package, version 24.0 (IBM Corporation, Armonk, NY, USA). Values are reported as means and standard deviations unless otherwise specified. An estimated sample size of 139 subjects was required to detect associations between continuous dependent variables (PSG-derived sleep parameters) and 15 predictors, considering a significance level of p < 0.05, a desired statistical power of 0.8, and an effect size (f2) of 0.15.(20)
Univariate linear regression analyses were initially performed to evaluate associations of resting lung function variables (FEV1/FVC, IC/TLC, RV/TLC, and DLCO in % of predicted values) and potential confounders (age, sex, BMI, AHI, parameters of nocturnal desaturation, comorbidities, psychotropic drug prescription, and alcohol intake) with PSG parameters that reflect sleep quality and architecture. (21-24) Thereafter, first-level multivariate analyses (backward stepwise method) were performed, including pulmonary function and PSG variables (AHI and parameters of nocturnal desaturation), as well as anthropometric and demographic variables, that showed p ≤ 0.10 in univariate models. If pulmonary function parameters remained as independent predictors of sleep performance in the first-level models, these multivariate analyses were further adjusted for presence of comorbidities, psychotropic drug prescription,(25) and alcohol intake on the day of PSG(26) if p was ≤ 0.10 in univariate models (final models). The significance level for retention of a variable in the multivariate model was set at p ≤ 0.05.
RESULTS The severity of COPD was widely distributed among the 181 patients included (post-bronchodilator FEV1 ranging from 25% to 128% of predicted): mild COPD, in 73 patients (40.3%); moderate COPD, in 78 (43.1%); and severe-very severe COPD, in 30 (16.6%). As expected, these participants presented with impaired ventilatory mechanics and gas exchange (DLCO) at rest according to the predicted values (Table 1).
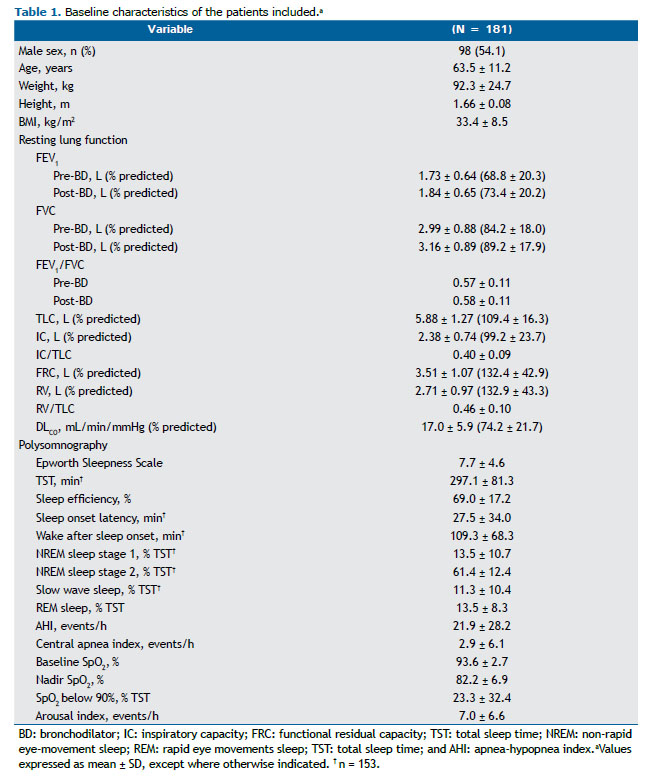
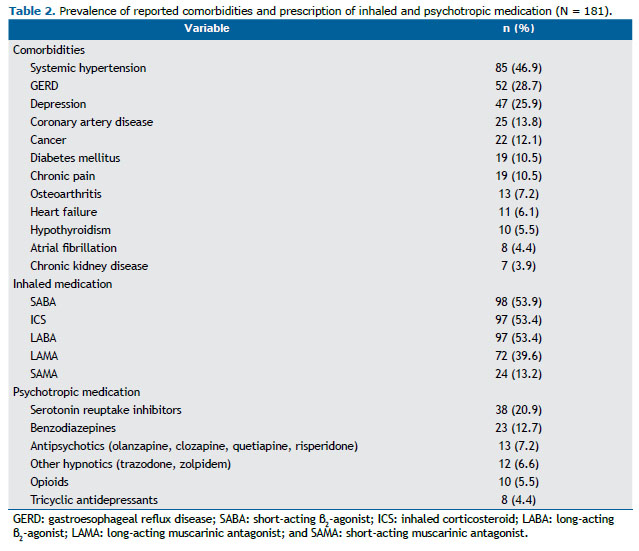
The subjects included in the study also showed reduced mean sleep efficiency when compared with historical controls, 144 (79.5%) of whom presenting reduced values (< 85%). PSG also unveiled a high proportion of OSA (116 subjects; 64.1%) and significant nocturnal desaturation (Table 1). The presence of OSA diagnosed by PSG was only related to lower slow wave sleep, expressed as % of TST (β = −4.212; R2 = 4%; p = 0.015). As anticipated, a high prevalence of comorbidities and prescription of psychotropic medication was reported (Table 2). Of 157 subjects in the sample, 22 (14.0%) reported alcohol consumption on the day of PSG.
Univariate linear regression analyses revealed that the selected resting lung function measures weakly correlated with PSG-derived parameters that reflect sleep quality and architecture (Table 3). In multivariate analyses, the pulmonary function parameters that remained as independent predictors of sleep efficiency, sleep onset latency, and rapid eye movement (REM) sleep (in % of TST) were FEV1/FVC, DLCO, and DLCO, respectively. As planned, these models were subsequently adjusted for the presence of comorbidities, psychotropic drug prescription, and alcohol consumption when these variables showed to be related to PSG-derived parameters of sleep quality in univariate analyses (data not shown). The resultant multivariate regression models depicting the final independent predictors are presented in Table 4.
DISCUSSION The major finding herein observed is that, after controlling for age, sex, AHI, nocturnal desaturation, comorbidities, psychotropic drug prescription, and alcohol consumption, selected parameters of resting lung function were weakly related to sleep quality and architecture in COPD: lower FEV1/FVC ratio was related to poorer sleep efficiency, whereas lower DLCO was associated with longer sleep onset latency and lower % of REM sleep.
PSG assessment of sleep quality is commonly found in research involving COPD.(3,6-8,12,27) The parameters that in fact correlate with subjective estimates of sleep quality, however, are controversial. We mainly analyzed variables that have been posited to correlate with subjective sleep quality as assessed via retrospective self-reported inventories or via ordinal scales included in prospective sleep diaries. Sleep efficiency and TST demonstrated to be significantly correlated with subjective sleep quality in a community-dwelling study including more than one thousand older adults regardless of sex.(21) The amount of slow-wave and/or REM sleep stages(22-24) and sleep onset latency(24) showed to be good predictors of subjective sleep satisfaction in smaller and older studies. Although several weak relationships were observed in univariate regressions, few lung function variables remained in the multivariate models only predicting sleep efficiency, sleep onset latency, and % of REM sleep, and all final multivariate models demonstrated low coefficients of determination (R2). This means that only a small proportion of the variance in the dependent variables could be predicted from the independent variables.Previous studies have also failed to find significant and/or robust associations between spirometric variables and sleep efficiency in COPD.(6,27) It has long been recognized, however, that due to the complexity of COPD, it is advisable to take into consideration physiological measures other than FEV1.(28) In fact, indexes of hyperinflation and gas trapping have proven to be more useful than has FEV1 in predicting cardinal symptoms of the disease, such as dyspnea and exercise intolerance,(29) as well as survival.(30) Accordingly, the IC/TLC ratio(12) and the length of the zone of apposition of the diaphragm(7) were associated with sleep efficiency. In the present study, however, which was adjusted to control for confounders, the significant relationships of gas trapping and lung hyperinflation with sleep efficiency observed in univariate regressions were no longer present in multivariate analyses. It is conceivable, therefore, that impaired respiratory mechanics and increased work of breathing(11) contributed only with a small fraction to the reduction of sleep efficiency. Accordingly, we recently showed that an evening dose of formoterol/aclidinium, when compared with placebo, improved overnight dynamic respiratory mechanics and inspiratory neural drive, but no positive effects on PSG outcomes (including sleep efficiency) were found.(31) A lower FEV1/FVC ratio is a sign of more severe airflow obstruction, possibly resulting in increased overnight symptoms (dyspnea, cough, and phlegm) and leading to frequent awakenings or difficulty in sleep onset.(3,4,32)
DLCO, in turn, persisted as the single pulmonary function variable independently related to sleep onset latency and % of REM sleep. Low resting DLCO has been reported to be associated with increased symptoms even in smokers without COPD and in patients with mild-moderate disease.(33) Consequently, DLCO might also be related to disturbed sleep because of a higher burden of overnight respiratory symptoms, such as nocturnal cough and dyspnea. In line with this premise, Chang et al.(32) found that the burden of COPD (particularly productive cough), evaluated through the COPD Assessment Test, was an independent factor for poor sleep quality. Recently, Lehmann et al.(34) found that the use of two bronchodilators (indacaterol/glycopyrronium) improved subjective sleep quality, lung function, and daily symptoms. The extent to which each of these mechanisms can improve sleep quality remains to be determined. From a physiological point of view, DLCO is a marker of alveolar-capillary membrane destruction from the early stages of COPD.(35) Low DLCO, therefore, reflects impaired vascular function across multiple anatomical sites, which was associated with poorer perceived sleep quality and % of REM sleep.(36) Interestingly, REM sleep is partially facilitated by nitric oxide,(37) a substance continuously synthesized by endothelial cells to maintain vascular homeostasis. Additional research is needed to explore the relationship between endothelial function/nitric oxide-mediated pathways and REM sleep in COPD.
From a clinical standpoint, detection and management of sleep disturbances seem relevant to reducing the burden of COPD.(9) Albeit to a small extent, selected lung function parameters reflecting the severity of airflow limitation and impairment in gas exchange were indeed related to sleep quality and architecture in COPD. It seems reasonable to consider these functional abnormalities in conjunction with other clinical signs of the disease (nocturnal wheezing, cough, and phlegm), COPD-related psychological distress, and polypharmacy in order to estimate the likelihood of poor sleep quality in individual patients.(9) Surprisingly, despite the high prevalence of diagnosed OSA (64.1%) in this sample of predominantly overweight participants, the presence of OSA, as well as the magnitude of overweight (BMI) and AHI, did not interfere with the observed relationships between lung function and sleep performance.
The main limitations in the present study are related to its cross-sectional design and retrospective nature. The former precludes strong mechanistic inferences. Although clinical information was carefully obtained from the institutional routine on the day of PSG, some additional potential clinical confounders may still have escaped. Anxiety and depression are highly prevalent(38) and associated with sleep disturbance in COPD(39): lack of assessment of these disorders by objective validated tools may have influenced our results. In addition, the fact that duration of COPD, exercise capacity, history of exacerbations, nocturnal/early-morning respiratory symptoms, adherence to prescribed psychotropic medications/duration of use, duration of alcohol consumption, and smoking on the day of PSG were not assessed restricted the possibility of further adjustment of the multivariate models. To our knowledge, although our sample represents the largest series to date in which sleep performance was objectively measured by overnight PSG and correlated with basic and advanced pulmonary function test results, we cannot rule out that if these additional potential confounders had been controlled, weaker relationship(s) could have been found.
In conclusion, selected pulmonary function variables reflecting the severity of airflow limitation and gas exchange efficiency, adjusted for some potential confounders, were weakly related to PSG outcomes in COPD patients. The direct contribution of these pathophysiological hallmarks of COPD to objectively measured sleep quality seems to be less important than it is generally thought, highlighting the complex pathogenesis of sleep disorders in this population of patients.
AUTHOR CONTRIBUTIONS RDM and DCB: study design; data collection and analysis; and drafting of the manuscript. ND, HD, and AFE: data collection and interpretation. MF, DEO, SF, and JAN: study design; data interpretation; and reviewing of the manuscript. All authors approved the final version of the manuscript.
REFERENCES 1. McNicholas WT. Impact of sleep in COPD. Chest. 2000;117(2 Suppl):48S-53S. https://doi.org/10.1378/chest.117.2_suppl.48S
2. Kinsman RA, Yaroush RA, Fernandez E, Dirks JF, Schocket M, Fukuhara J. Symptoms and experiences in chronic bronchitis and emphysema. Chest. 1983;83(5):755-761. https://doi.org/10.1378/chest.83.5.755
3. Cormick W, Olson LG, Hensley MJ, Saunders NA. Nocturnal hypoxaemia and quality of sleep in patients with chronic obstructive lung disease. Thorax. 1986;41(11):846-854. https://doi.org/10.1136/thx.41.11.846
4. Bellia V, Catalano F, Scichilone N, Incalzi RA, Spatafora M, Vergani C, et al. Sleep disorders in the elderly with and without chronic airflow obstruction: the SARA study. Sleep. 2003;26(3):318-323. https://doi.org/10.1093/sleep/26.3.318
5. Kessler R, Partridge MR, Miravitlles M, Cazzola M, Vogelmeier C, Leynaud D, et al. Symptom variability in patients with severe COPD: a pan-European cross-sectional study. Eur Respir J. 2011;37(2):264-272. https://doi.org/10.1183/09031936.00051110
6. Valipour A, Lavie P, Lothaller H, Mikulic I, Burghuber OC. Sleep profile and symptoms of sleep disorders in patients with stable mild to moderate chronic obstructive pulmonary disease. Sleep Med. 2011;12(4):367-372. https://doi.org/10.1016/j.sleep.2010.08.017
7. Krachman SL, Chatila W, Martin UJ, Permut I, D’Alonzo GE, Gaughan JP, et al. Physiologic correlates of sleep quality in severe emphysema. COPD. 2011;8(3):182-188. https://doi.org/10.3109/15412555.2011.560583
8. Calverley PM, Brezinova V, Douglas NJ, Catterall JR, Flenley DC. The effect of oxygenation on sleep quality in chronic bronchitis and emphysema. Am Rev Respir Dis. 1982;126(2):206-210.
9. Agusti A, Hedner J, Marin JM, Barbé F, Cazzola M, Rennard S. Night-time symptoms: a forgotten dimension of COPD. Eur Respir Rev. 2011;20(121):183-194. https://doi.org/10.1183/09059180.00004311
10. Budhiraja R, Siddiqi TA, Quan SF. Sleep disorders in chronic obstructive pulmonary disease: etiology, impact, and management. J Clin Sleep Med. 2015;11(3):259-270. https://doi.org/10.5664/jcsm.4540
11. O’Donnell DE, Laveneziana P, Webb K, Neder JA. Chronic obstructive pulmonary disease: clinical integrative physiology. Clin Chest Med. 2014;35(1):51-69. https://doi.org/10.1016/j.ccm.2013.09.008
12. Kwon JS, Wolfe LF, Lu BS, Kalhan R. Hyperinflation is associated with lower sleep efficiency in COPD with co-existent obstructive sleep apnea. COPD. 2009;6(6):441-445. https://doi.org/10.3109/15412550903433000
13. Marrone O, Salvaggio A, Insalaco G. Respiratory disorders during sleep in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2006;1(4):363-372. https://doi.org/10.2147/copd.2006.1.4.363
14. Luo YM, He BT, Wu YX, Yuan H, Xu J, Moxham J, et al. Neural respiratory drive and ventilation in patients with chronic obstructive pulmonary disease during sleep. Am J Respir Crit Care Med. 2014;190(2):227-229. https://doi.org/10.1164/rccm.201402-0302LE
15. Negewo NA, McDonald VM, Gibson PG. Comorbidity in chronic obstructive pulmonary disease. Respir Investig. 2015;53(6):249-258. https://doi.org/10.1016/j.resinv.2015.02.004
16. Iber C, Ancoli-Israel S, Chesson AL, Quan S. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications Westchester, IL: American Academy of Sleep Medicine; 2007.
17. Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, et al. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13(3):479-504. https://doi.org/10.5664/jcsm.6506
18. CarsKadon M, Dement W. Normal human Sleep: An Overview. In: Krieger M, Roth T, Dement W, editors. Principles and Practice of Sleep Medicine, 2nd ed. Philadelphia: WB Saunders; 1994. p. 16-25. https://doi.org/10.1016/B978-1-4160-6645-3.00002-5
19. Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255-1273. https://doi.org/10.1093/sleep/27.7.1255
20. Free Statistics Calculators, version 4.0 [homepage on the Internet]. Fullerton: Soper DS; c2006-2021; [cited 2020 Nov 1]. A-priori Sample Size Calculator for Multiple Regression. Available from: https://www.danielsoper.com/statcalc/calculator.aspx?id=1
21. Kaplan KA, Hirshman J, Hernandez B, Stefanick ML, Hoffman AR, Redline S, et al. When a gold standard isn’t so golden: Lack of prediction of subjective sleep quality from sleep polysomnography. Biol Psychol. 2017;123:37-46. https://doi.org/10.1016/j.biopsycho.2016.11.010
22. Westerlund A, Lagerros YT, Kecklund G, Axelsson J, Åkerstedt T. Relationships Between Questionnaire Ratings of Sleep Quality and Polysomnography in Healthy Adults. Behav Sleep Med. 2016;14(2):185-199. https://doi.org/10.1080/15402002.2014.974181
23. Hoch CC, Reynolds CF 3rd, Kupfer DJ, Berman SR, Houck PR, Stack JA. Empirical note: self-report versus recorded sleep in healthy seniors. Psychophysiology. 1987;24(3):293-299. https://doi.org/10.1111/j.1469-8986.1987.tb00298.x
24. Riedel BW, Lichstein KL. Objective sleep measures and subjective sleep satisfaction: how do older adults with insomnia define a good night’s sleep?. Psychol Aging. 1998;13(1):159-163. https://doi.org/10.1037/0882-7974.13.1.159
25. Roth T. Characteristics and determinants of normal sleep. J Clin Psychiatry. 2004;65 Suppl 16:8-11.
26. Ebrahim IO, Shapiro CM, Williams AJ, Fenwick PB. Alcohol and sleep I: effects on normal sleep. Alcohol Clin Exp Res. 2013;37(4):539-549. https://doi.org/10.1111/acer.12006
27. McSharry DG, Ryan S, Calverley P, Edwards JC, McNicholas WT. Sleep quality in chronic obstructive pulmonary disease. Respirology. 2012;17(7):1119-1124. https://doi.org/10.1111/j.1440-1843.2012.02217.x
28. Lange P, Halpin DM, O’Donnell DE, MacNee W. Diagnosis, assessment, and phenotyping of COPD: beyond FEV1. Int J Chron Obstruct Pulmon Dis. 2016;11 Spec Iss(Spec Iss):3-12. https://doi.org/10.2147/COPD.S85976
29. Albuquerque AL, Nery LE, Villaça DS, Machado TY, Oliveira CC, Paes AT, et al. Inspiratory fraction and exercise impairment in COPD patients GOLD stages II-III. Eur Respir J. 2006;28(5):939-944. https://doi.org/10.1183/09031936.06.00040506
30. Neder JA, Alharbi A, Berton DC, Alencar MC, Arbex FF, Hirai DM, et al. Exercise Ventilatory Inefficiency Adds to Lung Function in Predicting Mortality in COPD. COPD. 2016;13(4):416-424. https://doi.org/10.3109/15412555.2016.1158801
31. Domnik NJ, James MD, Scheeren RE, Ayoo GA, Taylor SM, Di Luch AT, et al. Deterioration of Nighttime Respiratory Mechanics in COPD: Impact of Bronchodilator Therapy. Chest. 2021;159(1):116-127. https://doi.org/10.1016/j.chest.2020.06.033
32. Chang CH, Chuang LP, Lin SW, Lee CS, Tsai YH, Wei YF, et al. Factors responsible for poor sleep quality in patients with chronic obstructive pulmonary disease. BMC Pulm Med. 2016;16(1):118. https://doi.org/10.1186/s12890-016-0281-6
33. Elbehairy AF, O’Donnell CD, Abd Elhameed A, Vincent SG, Milne KM, James MD, et al. Low resting diffusion capacity, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. J Appl Physiol (1985). 2019;127(4):1107-1116. https://doi.org/10.1152/japplphysiol.00341.2019
34. Lehmann S, Ringbæk T, Løkke A, Grote L, Hedner J, Lindberg E. A randomized trial to determine the impact of indacaterol/glycopyrronium on nighttime oxygenation and symptoms in patients with moderate-to-severe COPD: the DuoSleep study. Int J Chron Obstruct Pulmon Dis. 2019;14:199-210. https://doi.org/10.2147/COPD.S184127
35. Neder JA, Berton DC, Müller PT, Elbehairy AF, Rocha A, Palange P, et al. Ventilatory Inefficiency and Exertional Dyspnea in Early Chronic Obstructive Pulmonary Disease. Ann Am Thorac Soc. 2017;14(Supplement_1):S22-S29. https://doi.org/10.1513/AnnalsATS.201612-1033FR
36. Cooper DC, Ziegler MG, Milic MS, Ancoli-Israel S, Mills PJ, Loredo JS, et al. Endothelial function and sleep: associations of flow-mediated dilation with perceived sleep quality and rapid eye movement (REM) sleep. J Sleep Res. 2014;23(1):84-93. https://doi.org/10.1111/jsr.12083
37. Cespuglio R, Amrouni D, Meiller A, Buguet A, Gautier-Sauvigné S. Nitric oxide in the regulation of the sleep-wake states. Sleep Med Rev. 2012;16(3):265-279. https://doi.org/10.1016/j.smrv.2012.01.006
38. Putcha N, Drummond MB, Wise RA, Hansel NN. Comorbidities and Chronic Obstructive Pulmonary Disease: Prevalence, Influence on Outcomes, and Management. Semin Respir Crit Care Med. 2015;36(4):575-591. https://doi.org/10.1055/s-0035-1556063
39. Donovan LM, Rise PJ, Carson SS, Feemster LC, Griffith MF, Kapur VK, et al. Sleep Disturbance in Smokers with Preserved Pulmonary Function and with Chronic Obstructive Pulmonary Disease. Ann Am Thorac Soc. 2017;14(12):1836-1843. https://doi.org/10.1513/AnnalsATS.201706-453OC








