ABSTRACT
Objective: To evaluate the efficacy of a mindfulness-based treatment (MBT) for smoking cessation or reduction and compare it with that of cognitive behavioral therapy (CBT). Methods: This was a single-center randomized controlled clinical trial including 113 patients divided into two groups: MBT (n = 54) and CBT (n = 59). The interventions comprised eight 90-min sessions. The primary outcome was smoking cessation at 16 weeks after program initiation. Secondary outcomes included reduction in the mean number of cigarettes smoked/day at 16 weeks after treatment initiation, as well as smoking cessation and reduction in the number of cigarettes smoked/day at the last program session. Participants had to attend = 50% of the sessions to be included in the primary outcome analysis. An intention-to-treat analysis was also performed. Results: There was no difference between the groups regarding the primary outcome (30.4% in the MBT group vs. 31.6% in the CBT group, p = 0.68) or immediate abstinence rates (47.8% in the MBT group vs. 36.8% in the CBT group, p = 0.47). Both treatments were equally effective in reducing the number of cigarettes smoked/day at the last program session (a reduction of 93.33% [0-100%] in the MBT group and of 70% [33.3-100%] in the CBT group, p = 0.92) and at 16 weeks after program initiation (a reduction of 57.1% [0-100%] in the MBT group and of 70% [25-100%] in the CBT group, p = 0.49). Conclusions: MBT appears to be as effective as CBT for smoking cessation or reduction and can be an option for the treatment of tobacco use disorders in Brazil
(Brazilian Registry of Clinical Trials identifier: RBR-3w2scz [http://www.ensaiosclinicos.gov.br])
Keywords:
Mindfulness; Smoking cessation; Tobacco use disorder; Psychotherapy, group; Meditation; Cognitive behavioral therapy.
RESUMO
Objetivo: Avaliar a eficácia de um mindfulness treatment (MT, tratamento baseado em atenção plena) para a cessação ou redução do tabagismo e compará-la à da terapia cognitivo-comportamental (TCC). Métodos: Ensaio clínico controlado randomizado realizado em um único centro, com 113 pacientes divididos em dois grupos: MT (n = 54) e TCC (n = 59). As intervenções consistiram em oito sessões de 90 min cada. O desfecho primário foi a cessação do tabagismo 16 semanas após o início do programa. Os desfechos secundários foram a redução da média de cigarros fumados/dia em 16 semanas após o início do programa, bem como a cessação do tabagismo e redução do número de cigarros fumados/dia na última sessão do programa. Os participantes deveriam comparecer a ≥ 50% das sessões para que fossem incluídos na análise do desfecho primário. Foi também realizada uma análise por intenção de tratamento. Resultados: Não houve diferença entre os grupos quanto ao desfecho primário (30,4% no grupo MT vs. 31,6% no grupo TCC, p = 0,68) ou às taxas de abstinência imediata (47,8% no grupo MT vs. 36,8% no grupo TCC, p = 0,47). Ambos os tratamentos foram igualmente eficazes na redução do número de cigarros fumados/dia na última sessão do programa [redução de 93,33% (0-100%) no grupo MT e de 70% (33,3-100%) no grupo TCC, p = 0,92] e em 16 semanas após o início do programa [redução de 57,1% (0-100%) no grupo MT e de 70% (25-100%) no grupo TCC, p = 0,49]. Conclusões: A MT parece ser tão eficaz quanto a TCC para a cessação ou redução do tabagismo e pode ser uma opção para o tratamento do tabagismo no Brasil.
[Registro Brasileiro de Ensaios Clínicos – ReBEC; número de identificação: RBR-3w2scz (http://www.ensaiosclinicos.gov.br)]
Palavras-chave:
Atenção plena; Abandono do hábito de fumar; Tabagismo; Psicoterapia de grupo; Meditação; Terapia cognitivo-comportamental.
INTRODUCTION Cigarette smoking is the leading cause of preventable death in the world, associated with approximately 8 million annual deaths.(1) In Brazil, 9.8% of adults are current smokers,(2) and it is estimated that 428 people die every day because of smoking-related diseases.(3) In addition, smoking costs the country 56.9 billion Brazilian reals in health care and productivity loss.(3) Although the majority of smokers claim that they want to quit smoking, only 1-5% will achieve long-term cessation without professional help.(4,5)
Most currently available treatments in Brazil are based on cognitive behavioral therapy (CBT), including the smoking cessation treatment provided by the Brazilian Unified Health Care System. Pharmacotherapies can be used in association with CBT, particularly when there is a high degree of nicotine dependence. Although this strategy has yielded positive outcomes in the short term, the cessation rates tend to decrease significantly in the long term.(6)
Mindfulness is defined as the ability to pay attention to the present moment, on purpose and nonjudgmentally. (7) Mindfulness-based treatments (MBTs) were originally targeted at stress and chronic pain disorders.(8) Later, they were successfully used in the treatment of depression, anxiety,(9) eating disorders, and addictions,(10) and only recently have they been adapted for smoking cessation.(11)
MBT targeted to smoke cessation uses meditation techniques in order to increase the awareness of thoughts, feelings, and sensations, especially those related to craving. The goal is to teach participants to step out of the “automatic pilot” that leads to smoking and to cope with cravings until they no longer occur. In contrast to CBT, the focus is not on avoiding triggers, and substitutes are not used.(11)
The efficacy of MBT for smoking cessation has been shown to be similar to or, regarding long-term abstinence maintenance, even greater than that of CBT.(11-13) In addition, MBT has been shown to be associated with mood improvement, reduction in the urge to smoke, and reduction in abstinence symptoms.(14-16)
To our knowledge, there have been no studies evaluating MBT for smoking cessation in Brazil. The objective of the present study was to evaluate the efficacy of MBT in comparison with CBT for smoking reduction or cessation.
METHODS This was an open, single-center, randomized controlled clinical trial conducted between May of 2019 and January of 2020 at the Federal University of Paraná Hospital de Clínicas, located in the city of Curitiba, Brazil. The study was approved by the local research ethics committee (Protocol no. CAAE 02984118.8.0000.0096) and registered with the Brazilian Registry of Clinical Trials (ReBEC; primary identifier: RBR-3w2scz [http://www.ensaiosclinicos.gov.br]). All participants gave written informed consent before inclusion in the study.
Sample size calculation was performed on the basis of the results of a previous study.(11) We sought to include a total of 120 participants. Given that both interventions required active participation in the sessions, we decided to divide the participants in three groups for each intervention in order to keep the groups sufficiently small (20 participants per group). Participants were recruited through television, radio, Internet, and newspaper advertisements, as well as posters and flyers offering nonpharmacological treatment for smoking cessation.
The eligibility criteria were as follows: being ≥ 18 years of age; being a current smoker with an average consumption of at least 5 cigarettes per day and fewer than 3 months of abstinence in the past year; and being motivated to quit smoking within the next 30 days. The exclusion criteria were as follows: active substance dependence, including alcohol dependence; schizophrenia or panic disorder, diagnosed in accordance with the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition(17); illiteracy; and use of medication for smoking cessation, including bupropion, varenicline, and nicotine in any form.
Participants were randomized to intervention (MBT) or control (CBT) after stratification based on age (≥ vs. < 40 years old), sex, cigarettes smoked/day (> vs. ≤ 20), level of education (≥ complete high school education or < complete high school education), and Fagerström Test for Nicotine Dependence (FTND) scores (≥ 6 vs. < 6).
Both interventions comprised 90-min sessions twice weekly for four weeks. MBT sessions were based on a protocol used in a previous study.(11) The sessions covered theoretical concepts including associative learning, “automatic pilot,” and the understanding of thoughts as such (and not as absolute truths). Guided meditations common to several mindfulness programs were used, including the following: the body scan, loving-kindness meditation, sitting meditation, and mindfulness in daily activities. A technique to work with cravings “mindfully” (i.e., Recognize, Accept, Investigate, and Note what cravings feel like as they arise—RAIN) was also taught. Sessions were conducted by a qualified mindfulness instructor graduated from the Federal University of São Paulo Open Mind Center, located in the city of São Paulo, Brazil, a pulmonologist with experience in smoking cessation, and an undergraduate medical student. CBT was based on the Brazilian Unified Health Care System smoking cessation program,(18) also including exercises from the booklet Passos para uma Vida Livre do Tabaco (Steps to a Tobacco-Free Life)(19) and the American Lung Association Freedom from Smoking program. (20) The sessions were conducted by pulmonologists with experience in CBT for smoking cessation and an undergraduate medical student.
In addition to the in-person group sessions, home practice was suggested and additional materials were provided, including pamphlets, handouts, and recordings. Gradual reduction in cigarette consumption was encouraged, and the quit day was scheduled for the fourth session in both groups.
The primary outcome was smoking cessation at 16 weeks after program initiation. Secondary outcomes included reduction in the mean number of cigarettes smoked/day at 16 weeks after program initiation; smoking cessation and reduction in the mean number of cigarettes smoked/day at 4 weeks after program initiation (after the last session, i.e., immediate cessation); and correlation between attendance rates and percentage reduction in the mean number of cigarettes smoked/day.
Outcomes were assessed with standardized questionnaires administered by interviewers who were blinded to the intervention. Abstinence was confirmed by an exhaled carbon monoxide level of < 8 ppm.(21)
Only participants who attended at least 50% of the sessions were included in the primary analysis of the outcomes, because a minimum attendance rate was required for the learning process. An intention-to-treat analysis was performed, including all of the participants who attended at least the first session, as well as an analysis including only those who attended 75% of the sessions. Dropouts after randomization but before group assignment were excluded.
Participants who were lost to follow-up were considered nonabstinent. In accordance with standard practice in smoking cessation trials, a last-observation-carried-forward analysis was performed to quantify the number of cigarettes smoked/day.(22) In addition, attrition analysis was performed to assess systematic differences between participants with complete data and those lost to follow-up.
Data are reported as means ± SD for variables with normal distribution or as median (IQR) for variables with non-normal distribution. The unpaired t-test or the Mann-Whitney U test was used to compare continuous variables. Categorical variables were compared by Fisher’s exact test or the chi-square test. The paired t-test or the Wilcoxon test was used for within-patient comparisons. Spearman’s correlation coefficient was used to evaluate associations between variables. Differences were considered significant if p < 0.05. Data were analyzed with the R software, version 3.6.2 (The R Foundation for Statistical Computing, Vienna, Austria). Graphs and tables were created with GraphPad Prism software, version 9.2 (GraphPad Software Inc., San Diego, CA, USA).
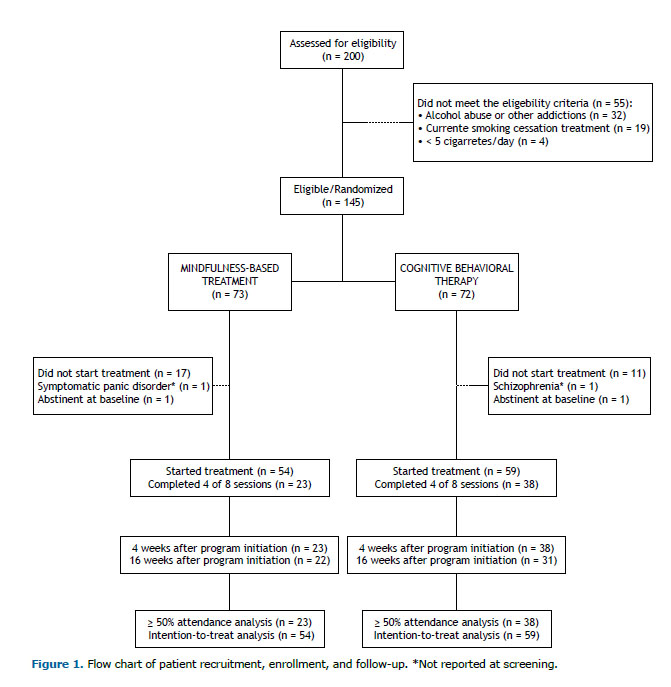
RESULTS Two hundred volunteers were assessed for inclusion in the study. Of those, 145 were considered eligible and randomized to MBT (n = 73) or CBT (n = 72). After randomization, 28 participants were excluded because they withdrew from the study before group assignment; 2 participants were excluded because of severe psychiatric disorders not reported during the screening phase (schizophrenia and panic disorder), and 2 were excluded because they were abstinent at the beginning of the trial. In total, 113 participants were included in the intention-to-treat analysis: 54 from the MBT group and 59 from the CBT group (Figure 1).
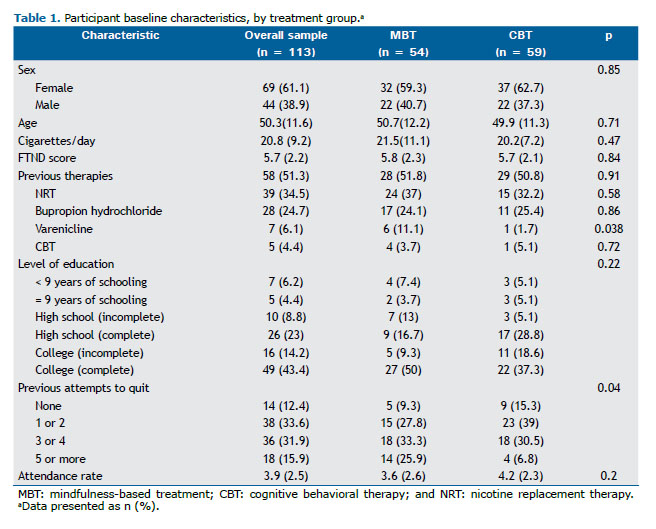
Both groups were well matched for sex, age, level of education, number of cigarettes smoked/day, and FTND scores. In the MBT group, the number of previous cessation attempts was higher, with 59% (vs. 32% in the CBT group) having tried to quit smoking three times or more. Most of the participants had previously undergone smoking cessation treatment, and previous use of varenicline was more common in the MBT group (Table 1).
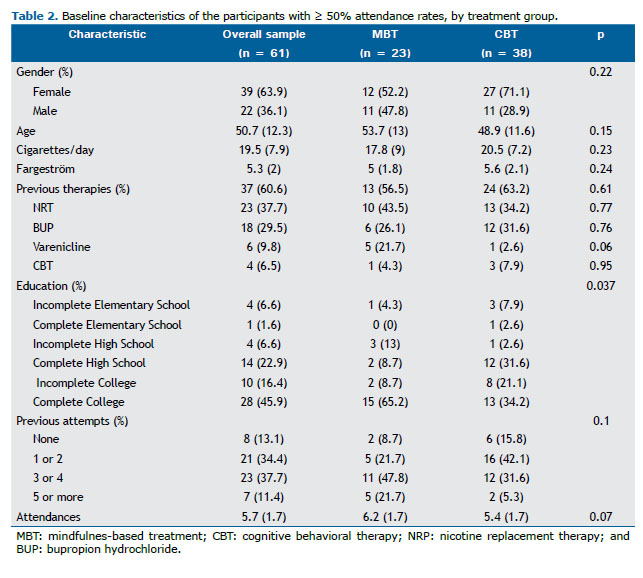
Baseline characteristics including sex, age, number of cigarettes smoked/day, and FTND scores remained similar when we evaluated the subgroup of participants with ≥ 50% attendance rates. Most (64%) of the participants were women, with a mean age of 51 ± 12 years, smoked > 20 cigarettes/day, and had a moderate degree of nicotine dependence (an FTND score of 5.3). There was a difference between the groups regarding the level of education (p = 0.037), the proportion of participants who had a college degree being higher in the MBT group than in the CBT group (65% vs. 34%; Table 2). Unlike what was observed in the intention-to-treat analysis, there were no differences between the groups regarding previous attempts to quit smoking and previous use of pharmacotherapy. In the MBT group, participants with attendance rates < 50% smoked more cigarettes/day before the study (17.8 ± 9 vs. 24.2 ± 11.8, p = 0.03) and had higher FTND scores (6.4 ± 2.4 vs. 5 ± 1.8, p > 0.001).
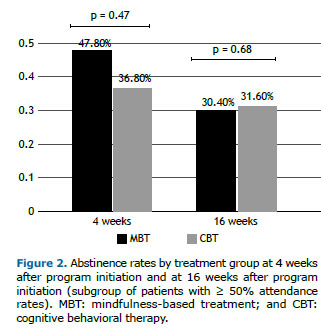
There were no significant differences between participants who were lost to follow-up and those with complete data regarding baseline characteristics. There was no difference between the groups regarding the primary outcome (smoking cessation at 16 weeks after program initiation) or immediate cessation rates (Figure 2).
Both treatments were effective (p < 0.001) in reducing the absolute number of cigarettes smoked/day at the end of treatment (a reduction of 19 cigarettes in the MBT group and of 15.5 cigarettes in the CBT group) and at 16 weeks after program initiation (a reduction of 14 cigarettes in the MBT group and of 10 cigarettes in the CBT group). There was no difference between the groups regarding this outcome (Figure 3). The relative reduction in the number of cigarettes smoked/day was = 93.3% (0-100%) in the MBT group vs. 70% (33.3-100%) in the CBT group at the end of treatment (p = 0.92) and 57.1% (0-100%) in the MBT group vs. 70% (25-100%) in the CBT group at 16 weeks after program initiation (p = 0.49).
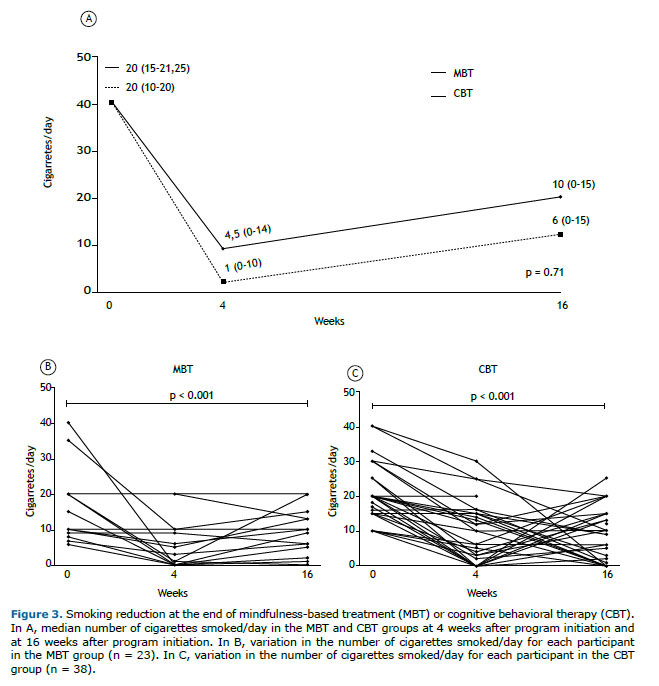
In the intention-to-treat analysis, there were no differences between the groups regarding abstinence at 16 weeks after program initiation (12.9% in the MBT group vs. 20.3% in the CBT group, p = 0.29) and at the end of treatment (20.4% in the MBT group vs. 23.7% in the CBT group, p = 0.66). However, when we analyzed participants with ≥ 50% attendance rates, both therapies were found to be more effective.
When we evaluated the participants who completed 75% of the programs (13 participants in the MBT group vs. 14 participants in the CBT group), cessation rates were found to be higher in both groups. The cessation rate was = 69% in the MBT group vs. 43% in the CBT group (p = 0.32) at the end of treatment and 38% in the MBT group vs. 43% in the CBT group (p = 0.85) at 16 weeks after program initiation.
There was a strong correlation between attendance at the sessions and the reduction in the proportion of cigarettes smoked/day at the last program session and at 16 weeks after program initiation (Figure 4).
DISCUSSION To our knowledge, this is the first randomized clinical trial evaluating the efficacy of MBT in comparison with CBT in Brazil. This is also the first study to adapt an MBT protocol for smoking cessation in our country. The main findings of the study are as follows: 1. no significant differences between the groups regarding abstinence rates at the end of the programs and at 16 weeks after program initiation; 2. no significant differences between the groups regarding the reduction in cigarettes smoked/day at the end of the programs and at 16 weeks after program initiation; 3. treatment efficacy comparable to that reported in the literature for both treatments and the evaluated outcomes; and 4. a strong correlation between session attendance rates and reduction in cigarettes smoked/day.
Long-term abstinence rates of approximately 16% have been achieved with the use of CBT alone (i.e., without any medication).(4) In Brazil, there is a lack of studies with adequate methodology evaluating CBT as a stand-alone treatment for smoking cessation. The available studies are small, are not randomized studies, are retrospective in nature, lack an objective verification of abstinence, or any combination of the four. Nevertheless, we chose to use CBT as a control intervention for several reasons: 1) It is the standard of care in our country; 2) It has demonstrated efficacy in epidemiological studies(23); 3) It has standardized materials; 4) Pulmonologists in Brazil are trained in administering CBT; and 5) The components of CBT are well matched to those of MBT, although the methods proposed for achieving cessation are different.
In a 2013 epidemiological study of CBT-based smoking cessation programs in the state of Paraná, Brazil,(23) 54% of the study participants attended the fourth (and last) treatment session; of those, 41% claimed to be abstinent (although this was not objectively determined). In addition, 61% were also receiving pharmacological treatment for smoking cessation. (23) In the present study, the MBT group achieved an abstinence rate of 20.4% at the end of the program in the intention-to-treat analysis and an abstinence rate of 47.8% when only participants with ≥ 50% attendance rates were evaluated. These results are comparable to those in the literature and are similar to those in the control group in our study.
In the present study, immediate cessation rates were = 47.8% in the MBT group and 36.8% in the CBT group (p = 0.25), whereas, in the study on which our MBT protocol was based, immediate cessation rates were = 36% in the MBT group and 15% in the CBT group (p = 0.063).(11) With regard to long-term abstinence, cessation rates were = 30.4% in the MBT group and 31.6% in the CBT group (p = 0.68) in the present study, whereas, in the aforementioned study, they were = 31% in the MBT group and 6% in the CBT group (p = 0.012).(11) Therefore, the results were comparable between the two MBT groups, whereas the CBT group in the present study performed better than did that in the aforementioned study.(11) In a larger clinical trial, in which either CBT or MBT was used in association with nicotine patches, immediate cessation rates were = 42.1% in the MBT group and 39.1% in the CBT group, and long-term cessation rates were = 19.4% in the MBT group and 23.8% in the CBT group. (12) Similar to our study, no significant differences were found between the two interventions in that study.(12) However, MBT showed benefits over CBT in promoting recovery from a lapse,(12) an outcome that was not evaluated in the present study.
Adherence to the programs had a direct impact on the results. This was corroborated by a positive correlation between attendance rates and the reduction in the number of cigarettes smoked/day, as well as by higher quit rates in participants with attendance rates ≥ 50% and 75%.
The main strength of our study lies in the fact that we were able to conduct a randomized controlled clinical trial with a diverse sample of smokers, including men and women of different age groups and levels of education. Another strength lies in the fact that the groups were matched for the duration of sessions and proposed home activities. In addition, assessing abstinence with a standardized questionnaire applied by an interviewer who was blinded to the intervention and performing exhaled carbon monoxide measurements made the outcomes more reliable. We also believe that the statistical analysis based on the attendance rates led to a better understanding of the results.
Our study has limitations that need to be addressed. The high number of dropouts reduced the power of the study and did not allow a detailed subgroup analysis. Withdrawal from the study before intervention assignment was higher in the MBT group, meaning that the number of participants in the MBT group was lower from the outset. This difference was accentuated when the subgroups with a minimum attendance rate of 50% were evaluated. This can be partially explained by the fact that the number of individuals with multiple cessation attempts, previous use of antismoking drugs, or a combination of the two was higher in the MBT group, a difference that disappeared in the subgroup with attendance rates ≥ 50%. Another hypothesis is that MBT might be easier to apply in individuals with a higher level of education, given that knowledge about meditation is more widespread in this population.(24) In our study, groups were well matched for level of education in the intention-to-treat analysis. However, the MBT group showed a higher prevalence of individuals with a higher level of education when we analyzed the subgroup of patients with ≥ 50% attendance rates. In addition, dropouts were higher in MBT group individuals who smoked more and had higher FTND scores, situations in which the association of pharmacotherapy is usually recommended.(25)
Missing data rates as a result of loss to follow-up in the long term were higher in the CBT group than in the MBT group (18% vs. 4%). Given that recurrence is common in this scenario, we believe that using the last-observation-carried-forward analysis might have underestimated the number of cigarettes consumed by some of those participants, therefore favoring the CBT group.
Another potential limitation is that the MBT instructors had little experience with the therapy applied. Although we had a certified mindfulness instructor and a pulmonologist with experience in smoking cessation, this was our first experience implementing a mindfulness-based protocol for smoking cessation. Professionals with more experience and maintenance sessions might have achieved even greater results.
In conclusion, the results of the present study indicate that MBT is as effective as CBT in promoting smoking cessation or reduction. If we consider that nicotine dependence is a very difficult-to-treat disease affecting millions of people of different socioeconomic, educational, and cultural backgrounds, we can understand how important it is to have different treatment options. Because MBT uses a strategy that is significantly different from CBT, it might become an interesting alternative, especially in individuals who have been unable to quit by using standard treatment.
Multicenter studies with larger samples are warranted to understand fully the potential of MBT for smoking cessation. Such studies will also be important in identifying the subsets of patients who will most likely benefit from this intervention, as well as in investigating the use of MBT in association with pharmacotherapy.
ACKNOWLEDGMENTS We would first like to thank the participants in this trial. We would also like to thank Giovanna Lemes Leão, Isabela De Bortoli, Rafaela Portiolli Tümmler, and Rebecca Saray Marchesini Stival for their role in assessing outcomes in this study. Finally, we would like to express our gratitude to Judson Brewer for kindly sharing his Mindfulness Training Manual for Smoking Cessation with us.
AUTHOR CONTRIBUTIONS MSA, LGS, and GMAP: conception and planning of the study; interpretation of evidence; drafting and revision of preliminary and final versions; and approval of the final version. NFP: conception and planning of the study; drafting and revision of preliminary and final versions; and approval of the final version. FMC, LM, DPN, and MC: planning of the study; revision of the final version; and approval of the final version. MHSO: interpretation of evidence; revision of the final version; and approval of the final version.
CONFLICT OF INTEREST None declared.
REFERENCES 1. World Health Organization [homepage on the Internet]. Geneva: World Health Organization; c2021 [updated 2021 Jul 26; cited 2021 Jun 26]. Tobacco. https://www.who.int/news-room/fact-sheets/detail/tobacco
2. Brasil: Ministério da Saúde, Secretaria de Vigilância em Saúde. VIGITEL BRASIL 2019, Vigilância de fatores de risco e proteção para doenças crônicas por inquérito telefônico. [monograph on the Internet]. Brasília: o Ministério; 2020 [cited 2021 Jun 26]. Available from: http://www.crn1.org.br/wp-content/uploads/2020/04/vigitel-brasil-2019-vigilancia-fatores-risco.pdf?x53725
3. Pinto, M, Bardach, A, Palacios, A, Biz, A, Alcaraz, A, Rodriguez, B, et al. Burden of smoking in Brazil and potential benefit of increasing taxes on cigarettes for the economy and for reducing morbidity and mortality. Cad Saude Publica. 2019;35(8):e00129118. https://doi.org/10.1590/0102-311x00129118
4. Fiore MC. US public health service clinical practice guideline: treating tobacco use and dependence. Respir Care. 2000;45(10):1200-1262. PMID: 11054899
5. Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99(1):29-38. https://doi.org/10.1111/j.1360-0443.2004.00540.x
6. Carlson LE, Taenzer P, Koopmans J, Bultz BD. Eight-year follow-up of a community-based large group behavioral smoking cessation intervention. Addict Behav. 2000;25(5):725-741. https://doi.org/10.1016/S0306-4603(00)00081-2
7. Terzi AM, Matos DP, Rodrigues ML, Demarzo M. Mindfulness in Education and Paulo Freire: a reflective approach. Interface (Botucatu). 2020; 24:e200015. https://doi.org/10.1590/interface.200015
8. Kabat-Zinn J, Lipworth L, Burney R. The clinical use of mindfulness meditation for the self-regulation of chronic pain. J Behav Med. 1985;8(2):163-190. https://doi.org/10.1007/BF00845519
9. Hofmann SG, Sawyer AT, Witt AA, Oh D. The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. J Consult Clin Psychol. 2010;78(2):169-183. https://doi.org/10.1037/a0018555
10. Warren JM, Smith N, Ashwell M. A structured literature review on the role of mindfulness, mindful eating and intuitive eating in changing eating behaviours: effectiveness and associated potential mechanisms. Nutr Res Rev. 2017;30(2):272-283. https://doi.org/10.1017/S0954422417000154
11. Brewer JA, Mallik S, Babuscio TA, Nich C, Johnson HE, Deleone CM, et al. Mindfulness training for smoking cessation: results from a randomized controlled trial. Drug Alcohol Depend. 2011;119(1-2):72-80. https://doi.org/10.1016/j.drugalcdep.2011.05.027
12. Vidrine JI, Spears CA, Heppner WL, Reitzel LR, Marcus MT, Cinciripini PM, et al. Efficacy of mindfulness-based addiction treatment (MBAT) for smoking cessation and lapse recovery: A randomized clinical trial. J Consult Clin Psychol. 2016;84(9):824-838. https://doi.org/10.1037/ccp0000117
13. Oikonomou MT, Arvanitis M, Sokolove RL. Mindfulness training for smoking cessation: A meta-analysis of randomized-controlled trials. J Health Psychol. 2017;22(14):1841-1850. https://doi.org/10.1177/1359105316637667
14. Cropley M, Ussher M, Charitou E. Acute effects of a guided relaxation routine (body scan) on tobacco withdrawal symptoms and cravings in abstinent smokers. Addiction. 2007;102(6):989-993. https://doi.org/10.1111/j.1360-0443.2007.01832.x
15. Leventhal AM, Zvolensky MJ. Anxiety, depression, and cigarette smoking: a transdiagnostic vulnerability framework to understanding emotion-smoking comorbidity. Psychol Bull. 2015;141(1):176-212. https://doi.org/10.1037/bul0000003
16. Sancho M, De Gracia M, Rodríguez RC, Mallorquí-Bagué N, Sánchez-González J, Trujols J, et al. Mindfulness-Based Interventions for the Treatment of Substance and Behavioral Addictions: A Systematic Review. Front Psychiatry. 2018;9:95. https://doi.org/10.3389/fpsyt.2018.00095
17. American Psychiatric Association. DSM-IV: Manual de Diagnóstico e Estatística das Perturbações Mentais. 4th ed. Lisboa: Climepsi Editores; 2002.
18. Brasil. Ministério da Saúde. Instituto Nacional de Câncer José Alencar Gomes da Silva (INCA). Deixando de Fumar sem Mistérios - Manual do Coordenador, 2nd ed. Rio de Janeiro: INCA; 2019. Available from: https://www.inca.gov.br/publicacoes/manuais/deixando-de-fumar-sem-misterio-manual-do-coordenador
19. Nunes DP, Lange MC, Zimmermann LM, Piovesan EJ, Scarinci IC. Intervenção para cessação do tabagismo em pacientes internados por AVC. Rev Psicol Saude. 2021;13(2):33-49. https://doi.org/10.20435/pssa.v13i2.1096
20. American Lung Association. Freedom From Smoking [homepage on the Internet]. Chicago, IL: American Lung Association; c2021 [cited 2021 Jun 26]. Resources - Information and Tools for Your Quit Journey. Available from: https://freedomfromsmoking.org/resources/
21. Gariti P, Alterman AI, Ehrman R, Mulvaney FD, O’Brien CP. Detecting smoking following smoking cessation treatment. Drug Alcohol Depend. 2002;65(2):191-196. https://doi.org/10.1016/S0376-8716(01)00162-4
22. Hedeker D, Mermelstein RJ, Demirtas H. Analysis of binary outcomes with missing data: missing = smoking, last observation carried forward, and a little multiple imputation. Addiction. 2007;102(10):1564-1573. https://doi.org/10.1111/j.1360-0443.2007.01946.x
23. Reichert J. Seção 1, Contexto Atual. In: Reichert J. 35 anos de história da luta contra o tabagismo no Paraná. Curitiba: Secretaria de Estado da Saúde do Paraná; 2015. p.118.
24. Macinko J, Upchurch DM. Factors Associated with the Use of Meditation, U.S. Adults 2017. J Altern Complement Med. 2019;25(9):920-927. https://doi.org/10.1089/acm.2019.0206
25. Ministério da Saúde, Instituto Nacional de Câncer, Coordenação de Prevenção e Vigilância. Abordagem e Tratamento do Fumante - Consenso 2001. Capítulo 1, Bases teóricas para as recomendações dos métodos de cessação de fumar. Rio de Janeiro: INCA; 2001. p. 16.







