TO THE EDITOR, Patients with severe asthma have worse anxiety and depression symptoms, functional capacity, physical activity levels, and quality of life compared to people without the disease.(1) In addition, this population often reports limitations when performing activities of daily living (ADL), such as doing chores, climbing stairs, going outdoors, or undertaking professional activities.(2) Studies have shown positive effects of add-on omalizumab therapy in patients with severe asthma, including improvements in asthma control, quality of life, and physical activity, while reducing the number of exacerbations and hospitalizations. (3) However, the impact of add-on omalizumab on ADL in patients with severe asthma has not yet been studied. We hypothesized that adults with severe stable asthma treated with add-on omalizumab therapy would report less limitation in ADL due to dyspnea. Thus, the aim of the present study was to compare the ADL limitations in subjects with severe asthma with and without add-on omalizumab therapy.
This real-life cross-sectional study included a convenience sample of patients diagnosed with severe asthma, according to the Global Strategy for Asthma Management and Prevention (GINA 2019),(4) with 18 years or more of age, under medical treatment for at least 6 months, clinical stability for at least 30 days (i.e., no symptom exacerbations, need for an oral corticosteroid course, or increment in asthma medication), and absence of limiting cardiovascular and/or musculoskeletal diseases. Subjects were excluded if they had a diagnosis of other pulmonary disease(s) aside from asthma. Recruitment took place by inviting patients followed up at the outpatient clinic of the University Hospital of Londrina (Brazil) to take part in a secondary study aiming to assess functional capacity in patients with asthma.(5) Data collection was carried out between April 2018 and June 2019. The study was approved by the Pitágoras-Unopar University Ethics Committee (protocol No. 3.060.314), and all participants signed an informed consent form.
The patients were assessed regarding sociodemographic data, the number of comorbidities, anxiety and depression symptoms,(6) exacerbations in the previous year, and use and dose of asthma medication. Anthropometric data, biomarkers (eosinophil and total Immunoglobulin E), the 7-item Asthma Control Questionnaire (ACQ - uncontrolled asthma if >1.50 points),(7) and lung function (Spirometer MicroLab 3500, Care Fusion®, Ireland)(8) were also assessed. The limitation to perform ADL was analyzed using the London Chest Activity of Daily Living (LCADL) scale,(9) which has four domains (personal care, domestic, physical activity, and leisure) distributed into 15 items of ADL, each scored from 0 to 5. The total score can vary from 0 to 75 points (sum of each item’s score), and the higher the score, the worse the limitation due to dyspnea in ADL.
In the statistical analysis, data distribution was verified by the Shapiro-Wilk test. Student’s T-test and the Mann-Whitney and Chi-squared tests were used to compare the two groups, according to the data distribution. Analysis of covariance (ANCOVA) was performed to adjust the LCADL score for sex due to cultural differences regarding ADL between men and women.(10) Spearman’s correlation coefficient was used to verify the correlation between the ACQ activity limitations question and the LCADL scores. The statistical software used was SPSS 22.0 (SPSS Inc., USA).
Forty-one patients met the inclusion criteria; however, four were excluded due to other pulmonary diseases aside from asthma, leaving 37 patients in the analysis. Most of the participants were middle-aged and overweight, and 21 (57%) presented uncontrolled asthma.(7) Fourteen patients (38%) had been receiving add-on omalizumab therapy for 30 (9-60) months. Most patients in the conventional therapy group seemed eligible for omalizumab since they had asthma symptoms that were inadequately controlled by inhaled corticosteroids (ICS) and total Immunoglobulin E levels between 30 and 1,500 IU/mL (Table 1).
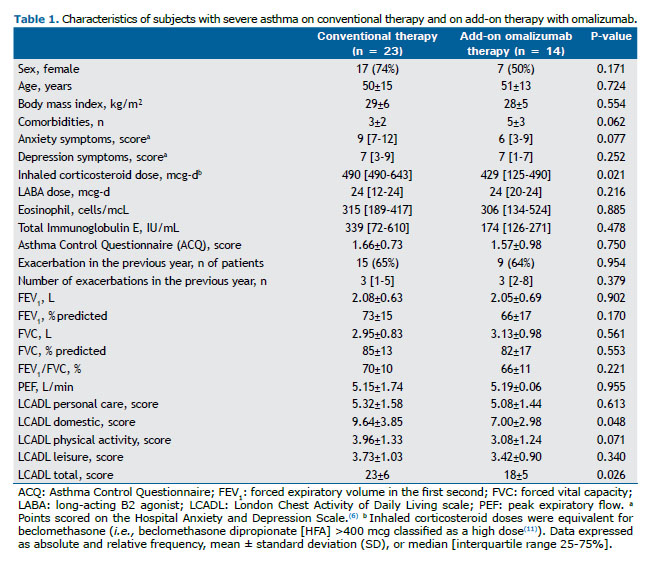
Both groups were similar regarding sex, age, body mass index, anxiety and depression, biomarkers, asthma control, exacerbations, and pulmonary function (p≥0.08 overall). There were also no differences in the number of patients who were employed or lived alone within the two groups (p = 0.790 and 0.135, respectively). The patients in the add-on omalizumab group used lower doses of ICS (p = 0.021) and had, on average, 2 more comorbidities than those in the conventional therapy group. Only one patient (omalizumab group) was in use of a long-acting muscarinic antagonist and oral corticosteroids as controller medication. The characteristics and comparisons of both groups are shown in Table 1.
The domestic domain and total LCADL were lower in the add-on omalizumab group, whereas the other domains were not significantly different between groups (Table 1). However, after the adjustment for sex (ANCOVA), which may be considered a confounder, the physical activity domain was also significantly lower in patients with add-on omalizumab therapy (mean [CI 95%] 4.02 [3.45-4.60] vs. 2.96 [2.16-3.76]; p = 0.041). The other domains were not significantly different in the ANCOVA (p≥0.134). The ACQ activity limitations question correlated with the personal care and leisure LCADL domains and with the total LCADL (r = 0.37, 0.48, and 0.40, respectively; p<0.030 overall).
To the best of our knowledge, this is the first study to compare the limitation due to dyspnea in ADL in patients with severe asthma undergoing conventional pharmacological therapy versus patients with add-on omalizumab therapy. The results showed that adults with severe asthma who received omalizumab therapy had less limitation in ADL reflected in the LCADL domestic and physical activity domains, as well as in the total score, despite having a higher number of comorbidities than the conventional therapy group (borderline statistical significance [p = 0.06] but clinically relevant).(12)
Omalizumab reduces the release of inflammatory mediators that trigger the downstream inflammatory cascade in allergic asthma and has been shown to enable a reduction in ICS dose.(3) This might have occurred in this study since patients in the omalizumab group were using lower ICS doses than patients in the conventional therapy group; however, we cannot establish cause and effect due to the study design. Omalizumab was the first biological therapy approved for asthma treatment and, at the end of 2019, it was included in the public health system in Brazil. Then, at the 97th CONITEC meeting, which occurred in May 2021, it was decided that another biological therapy would be incorporated for use in the country, mepolizumab. Therefore, further studies investigating the effects of different biological therapies on ADL are warranted, especially interventional studies under controlled conditions such as randomized controlled trials.
Limitations of this study include the small number of subjects enrolled in both groups and the cross-sectional design, which does not allow us to infer cause and effect. However, the application of the LCADL was blinded regarding the subjects’ treatment, and both the patients and pulmonologists were blinded concerning the study objectives. Additionally, we did not include patients who had exacerbations or increments in asthma medication in the last 30 days and excluded patients who had other pulmonary diseases aside from asthma, which could confound the results. Furthermore, our results corroborate previous studies that found positive effects with this add-on therapy in several health aspects of these patients and reflect real-life therapy.
In conclusion, adults with severe asthma undergoing add-on omalizumab therapy reported less limitation in activities of daily living than those who did not use this treatment, despite presenting more comorbidities. A randomized controlled trial may confirm the results of this real-life study.
AUTHOR CONTRIBUTIONS JMO: literature search, study design, data collection and analysis, manuscript preparation and review. ACN, FMCS, and FP: study design, review of manuscript. KCF: literature search, study design, data analysis, manuscript preparation and review.
REFERENCES 1. Neale J, Orme MW, Majd S, Chantrell S, Singh SJ, Bradding P, et al. A comparison of daily physical activity profiles between adults with severe asthma and healthy controls. Eur Respir J. 2020;56(1):1902219. https://doi.org/10.1183/13993003.02219-2019.
2. Mancuso CA, Sayles W, Robbins L, Phillips EG, Ravenell K, Duffy C, et al. Barriers and facilitators to healthy physical activity in asthma patients. J Asthma. 2006;43(2):137–43. https://doi.org/10.1080/02770900500498584.
3. MacDonald KM, Kavati A, Ortiz B, Alhossan A, Lee CS, Abraham I. Short- and long-term real-world effectiveness of omalizumab in severe allergic asthma: systematic review of 42 studies published 2008-2018. Exp Rev Clin Immunol. 2019;15(5):553–569. https://doi.org/10.1080/1744666X.2019.1574571.
4. Global Initiative for Asthma: Global strategy for asthma management and prevention, 2019. Available from: https://ginasthma.org.
5. Oliveira JM de, Spositon T, Cerci Neto A, Soares FMC, Pitta F, Furlanetto KC. Functional tests for adults with asthma: validity, reliability, minimal detectable change, and feasibility. J Asthma. 2020;6:1–9. https://doi.org/10.1080/02770903.2020.1838540.
6. Botega NJ, Zomignani MR, Garcia C, Pereira WA. Mood disorders among medical in-patients: a validation study of the hospital anxiety and depression scale. Rev Saúde Pública. 1995;29(5):355–63. https://doi.org/10.1590/S0034-89101995000500004.
7. Cardoso MN, Chong-neto HJ, Rabelo LM, Riedi CA, Rosário NA. Utility of Asthma Control Questionnaire 7 in the assessment of asthma control. J Bras Pneumol. 2014;40(2):171–4. https://doi.org/10.1590/S1806-37132014000200011.
8. Graham BL, Steenbruggen I, Barjaktarevic IZ, Cooper BG, Hall GL, Hallstrand TS, et al. Standardization of spirometry 2019 update. An official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med. 2019;200(8):e70–e88. https://doi.org/10.1164/rccm.201908-1590ST.
9. Pitta F, Probst VS, Kovelis D, Segretti NO, Leoni AMT, Garrod R, et al. Validation of the Portuguese version of the London Chest Activity of Daily Living scale (LCADL) in chronic obstructive pulmonary disease patients. Rev Port Pneumol. 2008;14(1):27–47. PMID: 18265916.
10. Hu S, Mu Z. Extended gender inequality? Intergenerational coresidence and division of household labor. Soc Sci Res. 2021;93:102497. https://doi.org/10.1016/j.ssresearch.2020.102497.
11. Pizzichini MMM, Carvalho-Pinto RM de, Cançado JED, Rubin AS, Cerci Neto A, Cardoso AP, et al. 2020 Brazilian Thoracic Association recommendations for the management of asthma. J Bras Pneumol. 2020;46(1):e20190307. https://doi.org/10.1590/1806-3713/e20190307.
12. Freitas PD, Xavier RF, McDonald VM, Gibson PG, Cordova-Rivera L, Furlanetto KC, et al. Identification of asthma phenotypes based on extrapulmonary treatable traits. Eur Respir J. 2020;2000240. DOI: 10.1183/13993003.00240-2020.


