ABSTRACT
Postemetic rupture of the esophagus, also known as spontaneous rupture or Boerhaave's syndrome, was first described by Herman Boerhaave in 1724. This is a severe disease that causes high mortality rates and is difficult to diagnose not only because it is rare but also because it is frequently confused with other severe clinical conditions, such as acute myocardial infarction, perforated peptic ulcer, and acute pancreatitis. Herein, we describe three cases of patients with this syndrome. Two underwent primary repair of the rupture, and one underwent esophagectomy followed by reconstruction. There was one death due to septic shock in the immediate postoperative period. The other two patients presented favorable long-term evolution.
Keywords:
Esophageal diseases; Rupture, spontaneous; Mediastinitis; Esophagectomy.
RESUMO
A ruptura pós-emética do esôfago, também chamada ruptura espontânea ou síndrome de Boerhaave, foi descrita pela primeira vez em 1724 por Herman Boerhaave. Trata-se de uma doença grave, de alta mortalidade e de difícil diagnóstico, tanto por ser rara como por ser freqüentemente confundida com quadros graves mais comuns, como o infarto agudo do miocárdio, a úlcera péptica perfurada e a pancreatite
aguda. Descrevemos, a seguir, três casos de pacientes com esta síndrome. Dois foram submetidos ao reparo primário da lesão e um foi submetido à esofagectomia com posterior reconstrução. Houve um óbito por choque séptico no pós-operatório imediato. Os outros dois casos tiveram boa evolução a longo prazo.
Palavras-chave:
Esofagopatias; Ruptura espontânea; Mediastinite; Esofagectomia.
IntroductionPostemetic rupture of the thoracic esophagus, known as Boerhaave syndrome, was first described in 1724 by Herman Boerhaave in a report on the case of the Dutch squadron admiral, Baron Jan von Wassenaer, whose death was caused by repetitive episodes of self-induced vomiting. The first successful repair of this condition did not occur until 1947 and was reported by Norman Barrett in London.(1)
Boerhaave syndrome produces an extremely severe clinical profile resulting from mediastinitis and sepsis that develop rapidly due to extravasation of digestive secretions and food into the mediastinum and pleural space. Diagnosis is often delayed, since it is a rare disease and is usually confused with other equally serious but more prevalent pathologies such as acute myocardial infarction, perforated peptic ulcer and acute pancreatitis.(1) These factors contribute to its high mortality rate, roughly 39%, despite current medical resources.(2) Here, we describe three cases that were recently treated at our facility.
Case reportsCase 1A 49-year-old male patient was admitted to the Emergency Room of the Walfredo Gurgel Hospital for surgical repair of a complicated umbilical hernia with intestinal obstruction. Approximately 10 h after the surgical repair, the patient presented intense chest pain, diaphoresis, hypotension and dyspnea, which was initially treated as acute myocardial infarction. A chest X-ray (Figure 1) revealed left pleural effusion, leading to the initial hypothesis of pulmonary embolism. A thoracocentesis revealed an accumulation of dark fluid, suggestive of gastric stasis in the left pleural cavity. During the directed interview, the patient revealed that he had vomited excessively during the entire hospital stay. An esophagoscopy revealed extensive impairment of the distal esophageal lumen, which was exuding a large quantity of secretion similar to that obtained in the thoracocentesis, obstructing proper visualization of the defect.
With the initial diagnosis of esophageal rupture, the patient was submitted to a left posterolateral thoracotomy, which revealed extensive impairment of the distal esophagus, with necrosis and severe mediastinitis. Since primary repair was not possible, a subtotal esophagectomy was performed, together with cervical esophagostomy and pleural drainage, followed by laparotomy and gastrostomy.
Postoperative evolution was complicated by respiratory failure, acute kidney failure and pleural empyema requiring repeat surgery. The patient required parenteral nutrition support and intensive care for thirty days, before being released from the hospital with pharyngogastric discontinuity. Nine months later, he underwent successful reconstruction of the esophageal tract using a reversed gastric tube.(3)
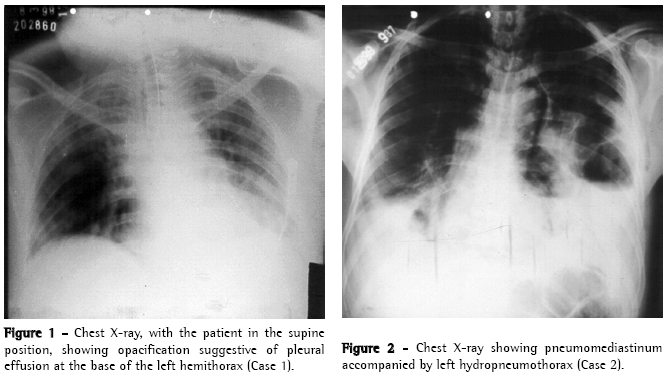
Case 2A 38-year-old male patient was admitted to the Emergency Room of the Walfredo Gurgel Hospital presenting spontaneous subcutaneous emphysema. During the directed interview, the patient reported alcoholism and recent episodes of vomiting after eating. He had no complaints of intense chest pain, indisposition or respiratory discomfort during the initial assessment. The physical examination and chest X-ray revealed pronounced subcutaneous emphysema in the chest and neck and condensation suggestive of pleural effusion in the left lung base (Figure 2). Fluid drawn during the thoracocentesis was dark in color with an aspect suggestive of gastric contents, leading to the suspicion of esophageal rupture, which was confirmed by the esophagogram.
A thoracotomy revealed extensive mediastinitis and a linear rupture in the distal lateral wall of the esophagus. The lesion was repaired using individual sutures of 3-0 polypropylene (Prolene®; Ethicon Endosurgery, Johnson & Johnson), as well as pleural reinforcement, complete pleural drainage and insertion of a gastrostomy feeding tube. However, on the following day, the patient evolved to refractory shock, multiple organ failure and death.
Case 3A 72-year-old male patient was admitted to the intensive care unit of another institution with abdominal and chest pain after incessant forceful vomiting. The initial chest X-ray revealed left pleural effusion. Cardiac enzymes and the electrocardiogram results were normal. A computed tomography scan of the chest revealed moderate pleural effusion and a small pneumothorax. The patient presented paleness, dyspnea, pale mucous membranes and strong peripheral pulse pressure. The blood pressure was 134/87 mmHg, the heart rate was 75 bpm, and the body temperature was 36.8 °C. The patient also presented a diminished left vesicular murmur, distended and painful abdomen without edema. The thoracocentesis revealed bloody fluid, presence of gram-positive germs and elevated amylase of 1,112 U/dL (normal 60-180 U/dL). The initial treatment consisted of intravenous hydration, analgesic treatment with morphine, antibiotic treatment and insertion of a nasogastric probe, through which 1500 mL of gastric contents were drained. The patient was transferred to our hospital and submitted to an esophagoscopy, which revealed esophageal rupture at the gastroesophageal junction. A thoracotomy was performed in the sixth intercostal space. A large amount of gastric fluid and rupture of the distal esophagus were found (Figure 3). An esophageal suture was inserted, followed by a diaphragmatic pedicle graft. The pleural cavity was thoroughly irrigated, and a water-sealed pleural drain was inserted. There were no postoperative complications. Two years and nine months later, the patient was asymptomatic and performing his professional duties.

DiscussionPerforation of the thoracic esophagus is one of the most serious digestive tract injuries in regard to mortality and morbidity. It causes a serious condition involving mediastinitis and sepsis due to the extravasation of digestive secretions and food particles into the mediastinum and pleural space. The prognosis mainly depends on the length of the delay between diagnosis and treatment, and, according to literature, mortality can be as high as 92% if the condition goes untreated and 60% if such treatment is delayed.(4)
Postemetic rupture of the esophagus accounts for 10% to 15% of all cases of thoracic esophageal perforation and is the third most common cause of this condition.(2) Diagnosis is particularly difficult and is usually delayed since it is a rare disease that can be confused with other serious clinical entities that are more common in emergency situations.(1) Clinical suspicion in relation to this entity is of utmost importance for proper diagnosis. Case 1 was initially diagnosed and treated as acute myocardial infarction and later, based on the clinical and radiological profile presented, as a pulmonary embolism. It was only after a thoracocentesis had been performed, with the discharge of suspicious pleural fluid, and the information that the patient had vomited excessively before the onset of thoracic discomfort became known that the possibility of an esophageal rupture was considered, which was confirmed in the thoracotomy.
Case 2 presented a clinical profile of spontaneous mediastinal and subcutaneous emphysema without the hemodynamic impairment and chest pain seen in Case 1, which led us to first consider spontaneous pneumomediastinum, which is a benign condition.(5,6) The presence of left pleural effusion accompanied by a suspicious thoracocentesis, together with a history of vomiting and alcoholism, prompted us to perform a esophagogram and led to the diagnosis of esophageal rupture.
Case 3 presented all of the classic signs of spontaneous esophageal rupture, which was confirmed by the elevated amount of amylase in the pleural fluid. The esophagoscopy not only confirmed the diagnosis but also revealed the location of the perforation, facilitating the choice of the type and location of the incision.
In almost all atraumatic esophageal ruptures, starting with Boerhaave's first case, there is concomitant vomiting, alcoholism and excessive food intake. Vomiting, particularly when repetitive, could lead to uncoordinated esophageal sphincter reflex opening. This dysfunction could result in a sudden, high magnitude increase in the intraluminal esophageal pressure, leading to rupture in the weakest region, which is the left lateral wall.(7)
The recommended treatment for this condition, when possible, is as follows: a thoracotomy with primary closure of the defect, with or without local reinforcement; mediastinal debridement and pleural drainage; gastrostomy to divert gastric secretions; and, occasionally, a jejunostomy for nutritional support.(8) Other recommended treatment options include the following: esophagectomy with or without immediate reconstruction of the pharyngogastric tract(9); esophageal exclusion using cervical esophagogastrostomy, cerclage and gastrostomy; and closure of the defect in conjunction with the use of a T-tube, similar to that used to drain bile ducts. These last procedures are usually performed in very serious situations with significant damage to the local tissue, in cases of late diagnosis or when primary repair is not possible.(2,10,11) Our first patient (Case 1) was submitted to a subtotal esophagectomy, which is a radical treatment, due to the extensive necrosis of the esophageal wall and the mediastinum, and this treatment might have contributed to his survival. In the other two patients, both of whom presented more favorable local conditions, primary repair was conducted. Nevertheless, one died as a result of refractory septic shock immediately following the surgery.
In conclusion, postemetic rupture of the esophagus is a serious condition whose diagnosis is usually neglected during the initial assessment. In the cases presented here, vomiting, left pleural effusion, thoracocentesis with aspiration of fluid suspected to be digestive secretions and elevated levels of amylase were the important factors in making the diagnosis and recommending the surgical treatment. Postoperative evolution is almost always complicated by organ failure and requires intensive care. High mortality and morbidity rates are to be expected.
AcknowledgmentsWe would like to thank Doctors Sami Assi João, Kaliandre Medeiros and Andréa Fernandes Oliveira for their participation in the management of Case 1.
References 1. Fell SC. Esophageal perforation. In: Pearson FG, Cooper JD, Deslauries J, Ginsberg RJ, Hiebert C, Patterson GA, et al., editors. Esophageal Surgery. New York: Churchill Livingstone, 2002. p. 615-36.
2. Jones WG, Ginsberg RJ. Esophageal perforation: a continuing challenge. Ann Thorac Surg. 1992;53(3):534-43.
3. Ximenes M, Silva RO, Vieira LF, Gregorcic A. The reversed gastric tube revisited: a useful esophageal replacement for benign disease. South Am J Thorac Surg. 1998;5(1):22-6.
4. Nemir P Jr, Wallace HW, Fallahnejad M. Diagnosis and surgical management of benign diseases of the esophagus. Curr Probl Surg. 1976;13(3):1-74.
5. Ralph-Edwards AC, Pearson FG. Atypical presentation of spontaneous pneumomediastinum. Ann Thorac Surg. 1994;58(6):1758-60.
6. Jougon JB, Ballester M, Delcambre F, Mac Bride T, Dromer CE, Velly JF. Assessment of spontaneous pneumomediastinum: experience with 12 patients. Ann Thorac Surg. 2003;75(6):1711-4.
7. Mackler SA. Spontaneous rupture of the esophagus; an experimental and clinical study. Surg Gynecol Obstet. 1952;95(3):345-56.
8. Whyte R I, Iannettoni MD, Orringer MB, Intrathoracic esophageal perforation. The merit of primary repair. J Thorac Cardiovasc Surg. 1995;109(1):140-4; discussion 144-6.
9. Orringer MB, Stirling MC. Esophagectomy for esophageal disruption. Ann Thorac Surg. 1990;49(1):35-42; discussion 42-3.
10. Abbott OA, Mansour KA, Logan WD Jr, Hatcher CR Jr, Symbas PN. Atraumatic so-called "spontaneous" rupture of the esophagus. A review of 47 personal cases with comments on a new method of surgical therapy. J Thorac Cardiovasc Surg. 1970;59(1):67-83.
11. Ximenes-Netto M, Silva RO, Fleury Jr I. Perfuração do esôfago. Rev Bras Cir. 1982;72(6):351-8.
_________________________________________________________________________________________
* Study conducted in the Surgery Department of the Health Sciences Center at the Rio Grande do Norte Federal University - CCS/UFRN - Natal (RN) Brazil; Walfredo Gurgel Hospital of the Secretary of Public Health of the State of Rio Grande do Norte, Natal, Brazil; and Thoracic Clinic of the Santa Lúcia Hospital, Brasilia (DF) Brazil.
1. Specialist of the Brazilian Society of Thoracic Surgery. Rio Grande do Norte Federal University - UFRN - Natal (RN) Brazil.
2. Thoracic Surgeon at Santa Lúcia Hospital, Brasilia (DF) Brazil.
3. Full Professor in the Surgery Department of the Health Sciences Center at Rio Grande do Norte Federal University - CCS/UFRN - Natal (RN) Brazil.
Correspondence to: Henrique José da Mota. Pneumocentro, Av. Campo Sales, 762, Tirol, CEP 59020-300, Natal, RN, Brasil.
Tel 55 84 3222-0303. Fax 55 84 3221-2280. E-mail: hjmota@ufrnet.br
Submitted: 11 June 2006. Accepted, after review: 08 September 2006.





