ABSTRACT
Objective: To determine whether COPD patients with diaphragmatic dysfunction present higher risk of mortality than do those without such dysfunction. Methods: We evaluated pulmonary function, diaphragm mobility and quality of life, as well as determining the Body mass index, airway Obstruction, Dyspnea, and Exercise capacity (BODE) index, in 42 COPD patients. The patients were allocated to two groups according to the degree to which diaphragm mobility was impaired: low mobility (LM; mobility ≤ 33.99 mm); and high mobility (HM; mobility ≥ 34.00 mm). The BODE index and the quality of life were quantified in both groups. All patients were followed up prospectively for up to 48 months in order to determine the number of deaths resulting from respiratory complications due to COPD. Results: Of the 42 patients evaluated, 20 were allocated to the LM group, and 22 were allocated to the HM group. There were no significant differences between the groups regarding age, lung hyperinflation or quality of life. However, BODE index values were higher in the LM group than in the HM group (p = 0.01). During the 48-month follow-up period, there were four deaths within the population studied, and all of those deaths occurred in the LM group (15.79%; p = 0.02). Conclusions: These findings suggest that COPD patients with diaphragmatic dysfunction, characterized by low diaphragm mobility, have a higher risk of death than do those without such dysfunction.
Keywords:
Pulmonary disease, chronic obstructive/mortality; Diaphragm; Exercise tolerance; Quality of life.
RESUMO
Objetivo: Verificar se indivíduos portadores de DPOC com disfunção diafragmática apresentam maior risco de mortalidade quando comparados àqueles sem disfunção diafragmática. Métodos: Foi avaliada a função pulmonar, a mobilidade diafragmática, a qualidade de vida e o índice conhecido como Body mass index, airway Obstruction, Dyspnea, and Exercise capacity (BODE) em 42 pacientes portadores de DPOC. Os pacientes foram alocados em dois grupos de acordo com a gravidade do acometimento da mobilidade do diafragma: grupo de baixa mobilidade (BM; mobilidade ≤ 33,99 mm) e grupo de alta mobilidade (AM; mobilidade ≥ 34,00 mm). O índice BODE e a qualidade de vida foram quantificados nos dois grupos. Todos os pacientes foram acompanhados prospectivamente por um período de até 48 meses a fim de identificarmos o número de óbitos decorrentes de complicações respiratórias devido a DPOC. Resultados: Dos 42 pacientes avaliados, 20 foram classificados no grupo BM, e 22 foram alocados no grupo AM. Não houve diferenças significativas quanto à faixa etária, hiperinsuflação pulmonar e fatores relacionados à qualidade de vida entre os grupos. Entretanto, o grupo BM apresentou maior pontuação no índice BODE em relação ao grupo AM (p = 0,01). O acompanhamento dos pacientes ao longo de 48 meses permitiu identificar quatro óbitos na população estudada, sendo todos os casos no grupo BM (15,79%; p = 0,02). Conclusões: Esses resultados sugerem que pacientes portadores de DPOC com disfunção diafragmática, caracterizada por uma baixa mobilidade do diafragma, apresentam maior risco de mortalidade quando comparados àqueles sem disfunção diafragmática.
Palavras-chave:
Doença pulmonar obstrutiva crônica/mortalidade; Diafragma; Tolerância ao exercício; Qualidade de vida.
IntroductionThe disease known as COPD is defined as a disease state characterized by airflow limitation that is not fully reversible.(1) Throughout the course of the disease, the increased airway resistance can lead to other pulmonary alterations, identified by an increase in static pulmonary volumes. This dynamic and progressive process, clinically recognized as air trapping, has been shown to be a limiting factor for diaphragmatic function in patients with COPD.(2)
In recent years, the evaluation of diaphragm mobility has become considered an additional parameter to identify diaphragmatic dysfunction in patients with chronic lung diseases.(3,4) Studies have shown that the impairment of diaphragm mobility might be associated with alterations in the principal pulmonary function parameters, such as FEV1.(2,5) The isolated determination of FEV1 has been recognized as a useful tool to predict health status, the frequency of exacerbations and the risk of mortality in patients with COPD.(6) However, the systemic manifestations of the disease are not reflected by this parameter. Therefore, a new multidimensional grading system-the Body mass index, airway Obstruction, Dyspnea, and Exercise capacity (BODE) index-has been proposed as a means of obtaining useful information regarding the prognosis of patients with COPD.(7) This index incorporates four main parameters of functional evaluation that reflect the pulmonary and systemic alterations of COPD: quantification of dyspnea; body mass index (BMI); exercise capacity based on the six-minute walk test (6MWT); and pulmonary function determined by the FEV1. The BODE index has been described as an important predictor of mortality (better that the isolated determination of FEV1)(7) and has been used as an indicator for the evaluation of pulmonary rehabilitation programs,(8) as a predictor of hospitalization(9) and as a predictor of worsening of quality of life.(10)
We recently described a classification of diaphragmatic dysfunction, based on the degree of diaphragm mobility, in which subgroups of patients, with varying degrees of diaphragm mobility, were characterized regarding functional parameters.(11) That study revealed that the individuals allocated to the ≤ 33.99 mm of diaphragm mobility group had greater sensation of dyspnea upon exertion and shorter six-minute walk distances (6MWDs) than did those allocated to the ≥ 34.00 mm of diaphragm mobility group. This classification of severity of diaphragmatic dysfunction was discriminatory regarding functional parameters. However, no characteristic regarding the incidence of mortality or the quality of life of these two subgroups of patients was described.
The principal objective of the present study was to evaluate whether the risk of death is higher for individuals with COPD and diaphragmatic dysfunction than for those with COPD and without diaphragmatic dysfunction. An additional objective was to compare two different levels of diaphragm mobility in terms of BODE index values and factors associated with quality of life.
MethodsOf a total of 277 patients under clinical follow-up treatment at the obstructive pulmonary diseases outpatient clinic of our institution, we selected 42 COPD patients (32 males) who met the criteria to participate in the study. The diagnosis of COPD was established based on the clinical history, on the physical examination findings and on the pulmonary function test results (postbronchodilator FEV1/FVC < 0.7).(1) All patients were former smokers, were receiving optimized clinical and therapeutic treatment and presented stable clinical conditions for at least 30 days before the evaluations (without symptom exacerbation or hospitalization).
The exclusion criteria were as follows: significant pulmonary response to bronchodilator use (increase in FEV1 greater than 15% of the baseline value or greater than 200 mL); inability to perform any of the requested tests; dependence on oxygen; presence of other known cardiopulmonary or hepatic diseases; and history of abdominal or thoracic surgery. The present study was approved by the ethics committee of the hospital, protocol no. 914/04, and all participants gave written informed consent.
The patients were submitted to an evaluation protocol comprising full pulmonary function testing, ultrasound measurement of diaphragm mobility, BODE index determination and quantification of factors associated with quality of life. The tests were performed within a maximum interval of two weeks, and all patients were clinically stable during the evaluation period.
The patients were allocated to two groups according to the degree to which diaphragm mobility was impaired: low mobility (LM; mobility ≤ 33.99 mm); and high mobility (HM; mobility ≥ 34.00 mm).(11) Patients in the LM group were considered individuals with diaphragmatic dysfunction. The BODE index and the quality of life were quantified in both groups. In addition, all patients were followed up prospectively for up to 48 months after the evaluation in order to determine the occurrence of death resulting from COPD-related respiratory complications. Furthermore, the patients were grouped according to the values obtained with the Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification system and the BODE index in order to compare these two indices in terms of their sensitivity and specificity as predictors of mortality. The patients were classified as low GOLD (stage I or II) or high GOLD (stage III or IV). With regard to the BODE index, the patients were classified as low BODE (quartile I or II) or high BODE (quartile III or IV). Individuals classified as high GOLD or high BODE had greater pulmonary and functional impairment and were therefore at a higher risk of death.(12)
The pulmonary function tests were performed in the pulmonary function laboratory of our institution with a whole-body plethysmograph (Collins GS II, Collins, Braintree, MA, USA). Spirometric parameters, static lung volumes, airway resistance and COPD were quantified in accordance with the technique recommended by the American Thoracic Society and the European Respiratory Society,(13) based on criteria of reproducibility and acceptability (variation of < 5%). The reference values adopted for spirometry were those described by Knudson et al.(14)
The measurement of diaphragm mobility was performed with an ultrasound device (Logic 500 Pro Series; General Electric Medical Systems, Milwaukee, WI, USA) in mode B. The evaluation method used in the present study quantified the craniocaudal displacement of the left branch of the portal vein in order to measure diaphragmatic displacement. This method was recently validated(15) and proved sensitive to detect small variations in the pattern of diaphragmatic movement with changes in body position.(16) A detailed description of this method has been reported in previous studies.(2,11,16)
The BODE index was used in order to predict the risk of death. To calculate the BODE index, the following were evaluated: BMI; FEV1 (expressed in percentage of predicted); sensation of dyspnea, quantified by the Modified Medical Research Council (MMRC) scale; and 6MWD, the 6MWT being performed in accordance with the recommendations of the American Thoracic Society.(17) The patients were given predetermined scores according to the parameter values obtained, using a table with scores ranging from 0 to 10. High BODE index values are associated with a greater risk of death.(7)
Quality of life was evaluated using the Saint George's Respiratory Questionnaire (SGRQ),(18) which has been translated into Portuguese and validated for use in Brazil.(19) This questionnaire is specific for respiratory diseases and comprises 76 questions distributed in three domains: symptoms, activity and impact. There are predetermined scores for each domain, and the total score corresponds to the sum of the scores of the three domains. The total score can range from 0 to 100. Lower values indicate better quality of life of the patient.
Statistical analysisAll data are presented as mean ± SD. The Kolmogorov-Smirnov test was used in order to verify the normal variance of the data. To compare the parameters between the two groups, we used the Student's t-test (parametric data) or the Mann-Whitney test (nonparametric data). Kaplan-Meier curve analysis was used in order to describe the distribution of survival for the groups, and statistical significance was evaluated using the log-rank test. We determined the sensitivity and specificity of diaphragm mobility, the BODE index and the GOLD classification as predictors of patient mortality. Because diaphragmatic dysfunction might be associated with a worse prognosis, the patients classified as LM were referred to as those who presented a positive test when calculating sensitivity and specificity.(20) The analyses were performed using the SigmaStat software, version 3.5 (Systat Software Inc., San Jose, CA, USA). The level of statistical significance was set at p < 0.05 for all tests.
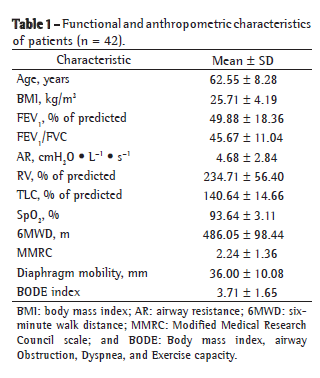
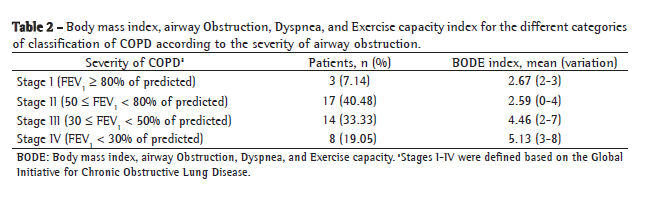
ResultsThe present study investigated 42 COPD patients (32 were male) with airway obstruction ranging from mild to severe (mean FEV1, 49.88 ± 18.36% of predicted). The functional and anthropometric characteristics of the patients are described in Table 1. The number of patients distributed in GOLD stages I-IV and the mean BODE index for each category are shown in Table 2.
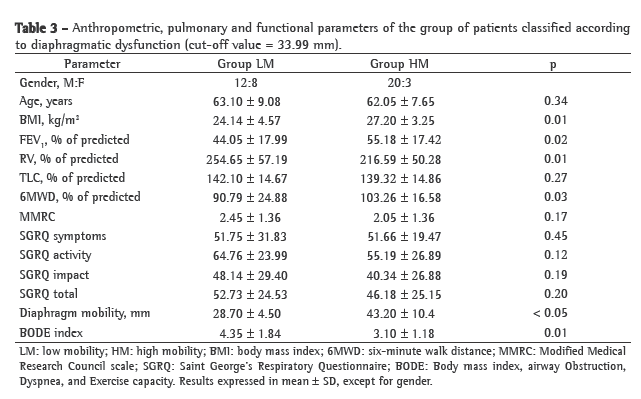
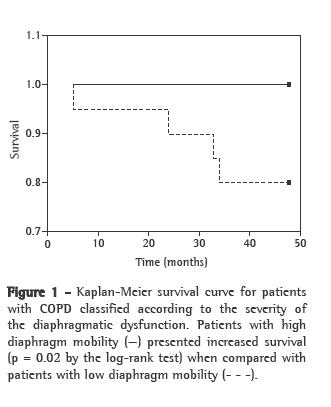
Of the 42 patients evaluated, 20 were classified as LM and 22 were classified as HM. Table 3 shows the results regarding the anthropometric, pulmonary and functional variables for the two groups of patients. The groups were comparable regarding age bracket and lung hyperinflation (assessed by TLC). There were no significant differences between groups LM and HM in terms of the score for any domain of the SGRQ. However, BODE index values were significantly higher in the LM group than in the HM group (p = 0.01). During the 48-month follow-up period, there were four deaths within the study sample; all of those deaths occurred in the LM group (15.79%) and resulted from COPD-related pulmonary complications. Figure 1 shows the Kaplan-Meier survival curve for the two diaphragm mobility groups. The log-rank test showed a significant difference between the two groups regarding the mortality rate (p = 0.02).
The sensitivity and specificity of the classification of diaphragmatic dysfunction as a predictor of mortality in the population studied were 100% and 58%, respectively. This calculation was also made for the classifications based on the GOLD or BODE indices. The results are summarized in Table 4.
 Discussion
DiscussionThe present study showed that COPD patients with diaphragmatic dysfunction (diaphragm mobility ≤ 33.99 mm) had higher BODE index values, indicating that these patients have a higher risk of death than do COPD patients without diaphragmatic dysfunction. The mortality rate, which was prospectively identified, was significantly higher in individuals with diaphragmatic dysfunction than it was in individuals without diaphragmatic dysfunction, supporting the use of the BODE index as a predictor of mortality. However, the degree of diaphragmatic dysfunction had no discriminatory power in relation to the quantification of quality of life.
A reduction in diaphragm mobility has been identified in patients with COPD and has been associated with a decline in pulmonary function parameters.(21) In a previous study, we demonstrated a relationship between diaphragmatic dysfunction and air trapping.(2) Other authors have also described an association between FEV1 reduction and impaired diaphragm mobility.(4,5) These results, taken together, strengthen the hypothesis that diaphragmatic dysfunction is related to the severity of the pulmonary disease.
Although FEV1 is considered an important marker of pulmonary obstruction severity, it does not adequately reflect the systemic manifestations of COPD.(22) Therefore, it has been proposed that another parameter, such as submaximal exercise capacity (evaluated by the 6MWT), should be evaluated in order to improve the understanding of the functional alterations in these individuals.(23) An association between diaphragmatic dysfunction and limited exercise tolerance has also been demonstrated.(11) Considering the results of these studies, which identified an association between limited diaphragm mobility and a decline in at least two of the determinants of the BODE index (FEV1 and exercise capacity),(2,11) we formulated the hypothesis that the more severe pulmonary and functional involvement observed in individuals with diaphragmatic dysfunction can reflect greater disease severity and, consequently, higher risk of death.
To test this hypothesis, the BODE index was calculated for the two groups of patients classified according to diaphragm mobility. The BODE index values were higher for the LM group patients, who also presented shorter 6MWDs, greater pulmonary obstruction and lower BMI than did those in the HM group. The only BODE index component that was not significantly different between the groups was sensation of dyspnea (evaluated using the MMRC scale). The higher BODE index values identified in the LM group patients suggest that such dysfunction is associated with a worse prognosis. The predictive power of the BODE index as an indicator of mortality risk was confirmed, the mortality rate being higher in the LM group patients, who were prospectively followed up for 48 months.
The sensitivity of diaphragm mobility for the identification of mortality was similar to that of the BODE index (100%). This indicates that the recognition of individuals with diaphragmatic dysfunction or the recognition of individuals with higher BODE index values were equally predictive of death within 48 months. However, the specificity of the BODE index (71%) was higher than was that of the diaphragm mobility classification (58%), which showed that a lower BODE index was more accurate than was the absence of diaphragmatic dysfunction as a predictor of survival. The sensitivity and specificity of the GOLD classification were lower than were those of the BODE index and of the diaphragm mobility classification.
These data corroborate results described previously, showing that the use of a single factor to indicate pulmonary severity (FEV1) does not seem to be the most appropriate means of understanding the systemic alterations and prognosis of patients with COPD.(6,8)
Some previous studies have investigated whether factors such as airway obstruction, exercise capacity, degree of dyspnea and quality of life, as well as symptoms of anxiety and depression, are independently associated with mortality in patients with COPD.(6,24-27) Although those studies demonstrated the relationship between these factors and mortality in COPD patients, none have analyzed this relationship using a variable that quantifies the dysfunction of the respiratory muscles. In the present study, we used the evaluation of diaphragm mobility as a parameter to characterize diaphragmatic dysfunction and observed that the presence of diaphragmatic dysfunction was also associated with higher BODE index values and higher mortality rates. The results of the present study support the use of both measurements as indicators of mortality, although the BODE index is more clinically viable because it consists of parameters easily measured in daily practice.
In recent decades, the quantification of factors related to quality of life has become the focus of researchers investigating the impact of COPD on the daily lives of individuals with COPD. Some studies have shown that there is gradual and progressive worsening of quality of life in patients with COPD as the pulmonary disease becomes more severe.(28,29) A recent study demonstrated an association between quality of life and the BODE index in individuals with severe COPD, showing that this index can be a reliable predictor of quality of life.(9) However, our results show that, although the classification of diaphragmatic dysfunction was concordant with the BODE index values, diaphragmatic dysfunction did not differentiate patients regarding factors related to quality of life. Therefore, the presence of diaphragmatic dysfunction does not seem to be associated with the deterioration of quality of life in patients with COPD.
Physical training of patients with COPD has been shown to have beneficial effects on some components of the BODE index, such as reduced sensation of dyspnea and increased exercise capacity.(30) In this sense, it was recently demonstrated that patients who participated in a program of physical training presented lower BODE index values and lower mortality rates than did those who did not participate in the program.(7) The authors suggested that the modification in BODE index values observed in the group submitted to the intervention provided relevant prognostic data, reflecting the longer survival of these patients. Considering that impaired diaphragm mobility is also associated with a higher risk of death, it is likely that specific training modalities that aim to improve diaphragm mobility and thoracic mobility can also have an impact on the BODE index and, consequently, on the survival of these patients. This hypothesis should be investigated in further studies.
A limitation of the present study was the small number of patients involved, which hindered the analyses in subgroups of patients classified according to the BODE and GOLD indices. Since the proportion of patients allocated to the different quartiles was relatively low, it was necessary to use a simpler division criterion, considering only two groups, that is, one group with greater impairment and another with lesser impairment. We believe that this had no major influence on the results, since there were, between the patients of these groups, significant differences in the parameters that determined the classification (BODE index and FEV1), which characterized the patients as having different levels of severity (data not shown). Despite these limitations, the present study provided an unprecedented contribution by presenting the use of a muscular evaluation parameter, which can be directly evaluated by means of prospective clinical monitoring, as a predictor of mortality.
The results of the present study suggest that COPD patients with diaphragmatic dysfunction, characterized by low diaphragm mobility, have a higher risk of death than do COPD patients without such dysfunction. Quality of life seems to be unrelated to the decline in diaphragmatic function in patients with COPD.
References 1. Fabbri L, Pauwels RA, Hurd SS; GOLD Scientific Committee. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: GOLD Executive Summary updated 2003. COPD. 2004;1(1):105-41; discussion 103-4.
2. Dos Santos Yamaguti WP, Paulin E, Shibao S, Chammas MC, Salge JM, Ribeiro M, et al. Air trapping: The major factor limiting diaphragm mobility in chronic obstructive pulmonary disease patients. Respirology. 2008;13(1):138-44.
3. Suga K, Tsukuda T, Awaya H, Takano K, Koike S, Matsunaga N, et al. Impaired respiratory mechanics in pulmonary emphysema: evaluation with dynamic breathing MRI. J Magn Reson Imaging. 1999;10(4):510-20.
4. Iwasawa T, Kagei S, Gotoh T, Yoshiike Y, Matsushita K, Kurihara H, et al. Magnetic resonance analysis of abnormal diaphragmatic motion in patients with emphysema. Eur Respir J. 2002;19(2):225-31.
5. Unal O, Arslan H, Uzun K, Ozbay B, Sakarya ME. Evaluation of diaphragmatic movement with MR fluoroscopy in chronic obstructive pulmonary disease. Clin Imaging. 2000;24(6):347-50.
6. Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS; GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163(5):1256-76.
7. Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005-12.
8. Cote CG, Celli BR. Pulmonary rehabilitation and the BODE index in COPD. Eur Respir J. 2005;26(4):630-6.
9. Ong KC, Earnest A, Lu SJ. A multidimensional grading system (BODE index) as predictor of hospitalization for COPD. Chest. 2005;128(6):3810-6.
10. Medinas-Amorós M, Alorda C, Renom F, Rubí M, Centeno J, Ferrer V, et al. Quality of life in patients with chronic obstructive pulmonary disease: the predictive validity of the BODE index. Chron Respir Dis. 2008;5(1):7-11.
11. Paulin E, Yamaguti WP, Chammas MC, Shibao S, Stelmach R, Cukier A, et al. Influence of diaphragmatic mobility on exercise tolerance and dyspnea in patients with COPD. Respir Med. 2007;101(10):2113-8.
12. Pitta F, Troosters T, Probst VS, Lucas S, Decramer M, Gosselink R. Potential consequences for stable chronic obstructive pulmonary disease patients who do not get the recommended minimum daily amount of physical activity. J Bras Pneumol. 2006;32(4):301-8.
13. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319-38.
14. Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127(6):725-34.
15. Toledo NS, Kodaira SK, Massarollo PC, Pereira OI, Mies S. Right hemidiaphragmatic mobility: assessment with US measurement of craniocaudal displacement of left branches of portal vein. Radiology. 2003;228(2):389-94.
16. Yamaguti WP, Paulin E, Shibao S, Kodaira S, Chammas MC, Carvalho CR. Ultrasound evaluation of diaphragmatic mobility in different postures in healthy subjects. J Bras Pneumol. 2007;33(4):407-13.
17. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111-7.
18. Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis. 1992;145(6):1321-7.
19. Sousa TC, Jardim JR, Jones P. Validação do questionário do Hospital de St. George na doença respiratória (SGRQ) em pacientes portadores de doença pulmonar obstrutiva crônica no Brasil. J Bras Pneumol. 2000;26(1):119-25.
20. Bewick V, Cheek L, Ball J. Statistics review 13: receiver operating characteristic curves. Crit Care. 2004;8(6):508-12.
21. Scott S, Fuld JP, Carter R, McEntegart M, MacFarlane NG. Diaphragm ultrasonography as an alternative to whole-body plethysmography in pulmonary function testing. J Ultrasound Med. 2006;25(2):225-32.
22. Gross NJ. Extrapulmonary effects of chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2001;7(2):84-92.
23. Rosa FW, Camelier A, Mayer A, Jardim JR. Evaluating physical capacity in patients with chronic obstructive pulmonary disease: comparing the shuttle walk test with the encouraged 6-minute walk test. J Bras Pneumol. 2006;32(2):106-13.
24. Oga T, Nishimura K, Tsukino M, Sato S, Hajiro T. Analysis of the factors related to mortality in chronic obstructive pulmonary disease: role of exercise capacity and health status. Am J Respir Crit Care Med. 2003;167(4):544-9.
25. Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121(5):1434-40.
26. Antonelli-Incalzi R, Pedone C, Scarlata S, Battaglia S, Scichilone N, Forestiere F, et al. Correlates of mortality in elderly COPD patients: focus on health-related quality of life. Respirology. 2009;14(1):98-104.
27. Funk GC, Kirchheiner K, Burghuber OC, Hartl S. BODE index versus GOLD classification for explaining anxious and depressive symptoms in patients with COPD - a cross-sectional study. Respir Res. 2009;10:1.
28. Graydon JE, Ross E. Influence of symptoms, lung function, mood, and social support on level of functioning of patients with COPD. Res Nurs Health. 1995;18(6):525-33.
29. Okubadejo AA, Jones PW, Wedzicha JA. Quality of life in patients with chronic obstructive pulmonary disease and severe hypoxaemia. Thorax. 1996;51(1):44-7.
30. Nici L, Donner C, Wouters E, Zuwallack R, Ambrosino N, Bourbeau J, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med. 2006;173(12):1390-413.
Study carried out at the Faculdade de Medicina da Universidade de São Paulo - FMUSP, University of São Paulo School of Medicine - São Paulo, Brazil and at the Santa Catarina State University, Florianópolis, Brazil.
Correspondence to: Celso Ricardo Fernandes Carvalho. Avenida Dr. Arnaldo, 455, sala 1210, CEP 01246-903, São Paulo, SP, Brasil.
Tel 55 11 3061-7317. Fax 55 11 3091-7462. E-mail: cscarval@usp.br or wellpsy@yahoo.com.br
Financial support: This study received financial support from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Coordination of the Advancement of Higher Education) and from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, National Council for Scientific and Technological Development).
Submitted: 28 March 2009. Accepted, after review: 4 August 2009.
About the authorsWellington Pereira dos Santos Yamaguti
Physical Therapist and Improvement Supervisor. Hospital Sírio Libanês, São Paulo, Brazil.
Elaine Paulin
Adjunct Professor. Santa Catarina State University, Florianópolis, Brazil.
João Marcos Salge
Pulmonologist. Department of Pulmonary Function, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo - HCFMUSP, University of São Paulo School of Medicine Hospital das Clínicas - São
Paulo, Brazil.
Maria Cristina Chammas
Director. Department of Ultrasound, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo - HCFMUSP, University of São Paulo School of Medicine Hospital das Clínicas - São Paulo, Brazil.
Alberto Cukier
Attending Pulmonologist. Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo - HCFMUSP, University of São Paulo School of Medicine Hospital das Clínicas - São Paulo, Brazil.
Celso Ricardo Fernandes de Carvalho
Professor. Universidade de São Paulo - USP, University of São Paulo - São Paulo, Brazil.








