ABSTRACT
Objective: To describe and to compare MIP and MEP in primigravidae and nulligravidae in the 20-29 year age bracket and paired by age. Methods: We included 120 primigravidae with low obstetric risk (5th-40th week of gestation) and 40 nulligravidae. All of the participants were of normal weight and none exercised regularly. All were recruited from the metropolitan area of Recife, Brazil. Measurements of MIP and MEP were obtained from RV and TLC, respectively, with a digital manometer. We used Student's t-test to compare the two groups, and we used multiple linear regression in order to determine whether group or chronological age correlated with MIP or MEP. Results: In the primigravida and nulligravida groups, the mean MIP values were 88.50 ± 16.52 cmH2O and 94.22 ± 22.63 cmH2O, respectively, (p = 0.08), whereas the mean MEP values were 99.76 ±18.19 cmH2O and 98.67 ± 20.78 cmH2O (p = 0.75). Gestational age did not correlate with MIP (r = −0.06; p = 0.49) or MEP (r = −0.11; p = 0.22). The relationship between chronological age and MIP/MEP did not differ between primigravidae and nulligravidae (angular coefficient = 0.028 and 0.453, respectively). Conclusions: Within this sample of women in the 20-29 year age bracket, the respiratory pressures of primigravidae remained stable during pregnancy and did not differ significantly from those of nulligravidae.
Keywords:
Pregnancy; Respiratory function tests; Muscle strength.
RESUMO
Objetivo: Descrever e comparar os valores de PImáx e de PEmáx em primigestas e nuligestas na faixa etária de 20-29 anos pareadas por idade. Métodos: Foram estudadas 120 primigestas de baixo risco obstétrico, da 5ª a 40ª semana gestacional, e 40 nuligestas, eutróficas, não praticantes de atividade física, provenientes da região metropolitana do Recife (PE). Os valores de PImáx e PEmáx foram obtidos, respectivamente, a partir do VR e da CPT através de um manovacuômetro digital. A comparação entre os grupos foi feita pelo teste t de Student, e a relação dos fatores grupo e idade cronológica sobre as pressões foi avaliada através de regressão linear múltipla. Resultados: No grupo de primigestas e nuligestas, a média de PImáx foi de, respectivamente, 88,5 ± 16,52 cmH2O e 94,22 ± 22,63 cmH2O (p = 0,08), enquanto a média de PEmáx foi de 99.76 ± 18,19 cmH2O e 98,67 ± 20,78 cmH2O (p = 0,75). Não houve correlação entre a idade gestacional e PImáx (r = −0,06; p = 0,49) ou PEmáx (r = −0,11; p = 0,22). A relação entre idade cronológica e PImáx/PEmáx não diferiu entre primigestas e nuligestas (coeficiente angular = 0,028 e 0,453, respectivamente). Conclusões: As pressões respiratórias de mulheres primigestas mantiveram-se estáveis durante o ciclo gestacional e não diferem significativamente dos valores das nuligestas na faixa etária de 20-29 anos.
Palavras-chave:
Gravidez; Testes de função respiratória; Força muscular.
IntroduçãoDesde que Black e Hyatt(1) descreveram em 1969 um método adequado e acessível para a avaliação da força da musculatura respiratória, diversos estudos têm sido realizados mundialmente com o intuito de estabelecer valores de referência adequados para esses parâmetros.(2-5) Esses estudos envolveram diversas populações e faixas etárias, como crianças, adolescentes, adultos e idosos. No entanto, relatos sobre o comportamento da força muscular respiratória durante o período gestacional ainda são escassos e realizados com amostras pequenas.(6-8)
Durante a gestação, o sistema respiratório é submetido a uma série de alterações fisiológicas com o intuito de adaptar-se às novas demandas de oxigênio maternas e fetais. Já nas primeiras semanas, ocorre um aumento no volume minuto em decorrência do aumento do volume corrente, uma vez que a frequência respiratória não sofre uma alteração significativa.(8)
Mudanças também ocorrem tanto na configuração da caixa torácica quanto nos volumes estáticos pulmonares. A elevação de 4-5 cm do diafragma leva a um aumento em torno de 2 cm dos diâmetros transverso e anteroposterior, com consequente queda de 300-500 mL da capacidade residual funcional (CRF).(7-9) A diminuição da CRF é consequência tanto da diminuição no volume de reserva expiratório (100-300 mL) quanto do volume residual (200-300 mL). No entanto, não há alteração significativa da capacidade pulmonar total, uma vez que há um aumento da capacidade inspiratória (100-300 mL).(10)
Apesar de as alterações de volumes e as capacidades pulmonares, bem como as modificações anatômicas da caixa torácica durante a gestação estarem bem documentadas na literatura, as investigações sobre os valores da força muscular em gestantes são escassas e com algumas limitações metodológicas. Além disso, existem situações clínicas específicas envolvendo o sistema respiratório durante a gestação, como no caso das doenças neuromusculares, em que há necessidade do acompanhamento da força muscular respiratória durante o pré-natal.(11,12)
Diante da necessidade de se estudar o comportamento da força muscular respiratória no período gestacional, o presente estudo teve como objetivo descrever os valores de PImáx e PEmáx em uma amostra de primigestas e comparar tais resultados com valores encontrados em uma amostra de nuligestas.
MétodosEste é um estudo com delineamento transversal, cuja coleta de dados ocorreu no período entre janeiro de 2008 e março de 2009, no Ambulatório da Mulher do Instituto de Medicina Integral Professor Fernando Figueira, na cidade do Recife (PE).
A amostra, selecionada sequencialmente, incluiu 120 primigestas da 5ª a 40ª semana gestacional e 40 nuligestas provenientes da zona urbana da cidade de Recife e teve como critérios de inclusão faixa etária de 20 a 29 anos, não praticantes de atividades físicas, eutróficas e de baixo risco obstétrico, no caso das gestantes. Adotou-se o índice de massa corpórea (IMC) adequado para a idade gestacional, de acordo com o método proposto por Atalah et al.,(13) enquanto, para as nuligestas, consideraram-se como adequados valores de IMC entre 20 e 25 kg/m2.(14) Foram considerados como critérios de exclusão a presença de deformidades na coluna ou na caixa torácica, história de tabagismo ou de pneumopatias, presença de gripes, resfriados ou doenças neuromusculares e incapacidade de compreender ou realizar os procedimentos.
O presente estudo obteve aprovação do comitê de ética e pesquisa em seres humanos do referido hospital sob o número de protocolo 986/2007. Todas as mulheres que aceitaram participar assinaram o termo de consentimento livre e esclarecido segundo critérios prescritos pela resolução 196/96 do Conselho Nacional de Saúde.
Todas as gestantes foram submetidas a uma avaliação prévia que consistia na obtenção de dados pessoais, sociodemográficos e antropométricos. A idade gestacional foi calculada a partir da data da última menstruação (DUM) ou por ultrassonografia de 1º trimestre quando havia dúvida sobre a DUM.
Os valores de PImáx e PEmáx eram obtidos, sempre no horário da manhã, utilizando-se um manovacuômetro digital com válvula unidirecional (modelo MVD300; G-MED®, Porto Alegre, Brasil) com escala de fundo de 1-480 cmH2O e calibrado sistematicamente. As mensurações eram realizadas com a gestante sentada em uma cadeira com encosto, com os pés apoiados no chão, quadris e joelhos a 90°, utilizando um clipe nasal e respirando através de uma boquilha oval (2,8 × 0,7 cm) com um bocal intermediário com um orifício de fuga de 2 mm.
Com uma das mãos, a gestante segurava a boquilha firmemente, pressionando-a contra os lábios, evitando vazamento perioral durante a realização das manobras, e apoiava as bochechas com a outra mão.
Antes da realização das manobras, cada voluntária recebia explicação e demonstração quanto à utilização do manovacuômetro para a correta mensuração das pressões respiratórias máximas, e eram realizadas três manobras prévias à coleta, que eram consideradas como teste.
A PImáx foi obtida a partir do VR, para a qual a gestante era orientada a expirar até o VR e então inspirar profundamente no manovacuômetro. A PEmáx foi obtida a partir da CPT, para a qual a gestante era orientada a inspirar até a CPT e então expirar profundamente no manovacuômetro.
O mesmo encorajamento verbal era fornecido a cada gestante, bem como um feedback visual através do monitor do aparelho para que se atingisse o esforço máximo no momento da mensuração das pressões respiratórias.
Para cada volume, eram realizadas pelo menos três manobras com intervalo de 1 min entre uma e outra. Registravam-se apenas as manobras estáveis por pelo menos 1 s, sem vazamento de ar e com variação de 10% entre os valores, sendo o maior valor selecionado para a análise final.
O nível de atividade física foi averiguado através do Questionário Internacional de Atividade Física, versão 8, na forma curta, com resultados expressos em min/semana.(15)
Os valores dos grupos foram expressos em médias e desvios-padrão, sendo as diferenças entre dois grupos avaliadas pelo teste t de Student e, entre mais de dois grupos, por ANOVA. O coeficiente de correlação de Pearson foi empregado para avaliar a associação entre as pressões respiratórias e a idade gestacional. O efeito da idade e do grupo sobre as pressões foram avaliados através do ajuste de modelos de regressão linear.
Para a análise estatística, foi utilizado o software Minitab versão 14.0 (Minitab Inc., State College, MA, EUA), e todos os testes foram aplicados com nível de confiança de 95%, considerando-se significativo o valor p < 0.05.
ResultadosA idade média do grupo de primigestas e de nuligestas foi de, respectivamente, 23,34 ± 2,7 anos e 24,05 ± 3,02 anos, sem diferença significativa.
Os grupos estudados foram homogêneos quanto ao nível de atividade física e a média dos valores de PImáx e PEmáx, os quais não diferiram significativamente entre as primigestas e as nuligestas (Tabela 1).
Também não houve diferenças significativas entre as médias das pressões nos trimestres do grupo das primigestas e entre as nuligestas (Tabela 2).
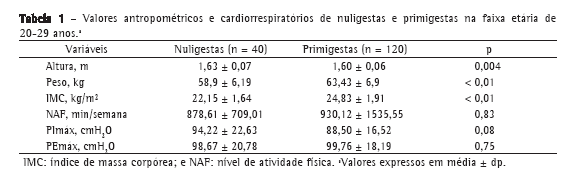
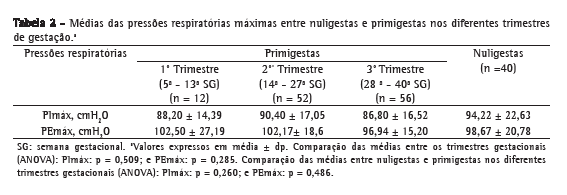
A idade gestacional média do grupo de primigestas foi de 27,3 ± 8,79 semanas. Além disso, essa variável não esteve associada com os valores de PImáx (r = −0,06; p = 0,49) ou de PEmáx (r = −0,11; p = 0,22).
Conforme pode ser observado nas Figuras 1 e 2, não houve variações significantes de PImáx ou PEmáx, tanto para primigestas como para nuligestas, com o aumento da idade. Também não houve diferenças estatisticamente significativas entre as médias das pressões das nuligestas e das primigestas para cada idade estudada.
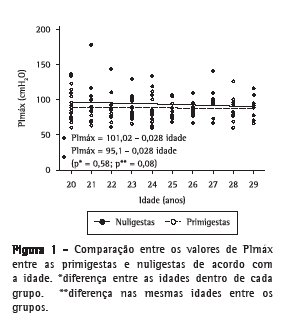
 Discussão
DiscussãoOs valores de PImáx e PEmáx no grupo de primigestas foram semelhantes e mantiveram-se semelhantes aos valores das nuligestas na faixa etária estudada. Tais achados adicionam mais informações ao comportamento das pressões respiratórias na gestação, pois, ao contrário das expectativas convencionais,(16) as adaptações morfofisiológicas respiratórias impostas pela gestação não implicam prejuízo à geração da força muscular respiratória. Embora a diferença dos valores de PImáx aqui encontrada (5,72 cmH2O; 6,07%) aproximou-se do nível de significância estatística, uma diminuição da pressão respiratória dessa magnitude não pode ser traduzida como reflexo de uma redução da força muscular inspiratória,(17,18) podendo ser justificada pelo menor volume residual pulmonar encontrado nessa população.
Os resultados encontrados podem ser justificados pelo funcionamento biomecânico respiratório, pois a redução na complacência da parede torácica associada ao aumento da pressão abdominal no final da expiração, em virtude do crescimento uterino, diminui a CRF e altera a posição de repouso do sistema respiratório.(7,19) Consequentemente, com a elevação do diafragma, a área de aposição aumenta em relação ao gradil costal, o que incrementa sua capacidade de gerar tensão.(6)
Associado a isso, há uma diminuição da complacência abdominal, o que favorece o controle da descida diafragmática e a manutenção de suas fibras musculares em uma posição vantajosa de comprimento-tensão. Além disso, a pressão transdiafragmática não se altera,(7,16) e estudos(7,16,20) mostraram um aumento da excursão diafragmática e uma contribuição equitativa da musculatura intercostal e diafragmática para o volume corrente, o que facilita a manutenção da força muscular.
Compartilhando com todos esses ajustes fisiológicos, a manutenção da complacência pulmonar e a diminuição da resistência das vias áreas durante a gestação contribuem para a diminuição da carga imposta aos músculos respiratórios, auxiliando sua atuação, pois não é imposto um aumento no trabalho respiratório.(21,22) Portanto, todas essas adaptações favorecem a mecânica muscular, mantendo a eficiência da musculatura inspiratória, apesar da progressiva distorção toracoabdominal imposta pela gestação.
Da mesma forma, a mecânica expiratória também parece adaptar-se. Poder-se-ia supor que a distensão abdominal imposta pela gestação resultasse em uma diminuição nos valores de PEmáx, interferindo na geração de força expiratória. No entanto, os resultados do presente estudo não confirmaram tal suposição. De acordo com um grupo de autores,(23) a manutenção da força tensional abdominal na gestação poderia ser explicada através da função neuromuscular. O músculo, ao ser submetido a um alongamento prolongado, reage com a mudança no seu comprimento por adição no número de sarcômeros. Esse processo, conhecido como miofibrilogênese,(24) facilita a sobreposição dos filamentos de actina e miosina aumentando a tensão máxima gerada pelo músculo.
Assim, diante do exposto, associado ao fato de que as pressões mantiveram-se constantes durante toda a gestação, parece que as adaptações morfológicas, fisiológicas e biomecânicas vigentes no sistema respiratório ajudam a minimizar as progressivas mudanças na forma e na configuração do abdome, do diafragma e da parede torácica, conseguindo, dessa forma, que a força muscular respiratória seja mantida.
Como esperado, a idade não influenciou os valores de pressão respiratória, uma vez que houve controle de uma faixa etária de 10 anos para homogeneizar a análise dos resultados entre os grupos estudados, excluindo gestantes adolescentes.
Embora tenha havido diferenças de altura encontrada entre os grupos, a similaridade nas pressões respiratórias entre eles indicam que esse fator não influenciou os valores encontrados, o que é compatível com resultados da literatura, os quais também não mostraram interferência desse parâmetro nas pressões.(1-3,5)
Os valores encontrados na população gestante apresentaram pouca diferença em relação aos de outros estudos que obtiveram valores médios de PImáx de 76,(22) 86(6) e 88(7) cmH2O e de PEmáx de 104,(22) 94(6) e 93(7) cmH2O. Da mesma forma, um grupo de autores,(8) avaliando as pressões em gestantes dispneicas e não dispneicas, encontraram uma média de valores de PImáx entre 78 ,0 e 81,9 cmH2O e uma média de PEmáx entre 97,2 e 106,8 cmH2O, não havendo diferenças significativas entre os grupos. No entanto, esses estudos envolveram amostras pequenas (entre 8 e 23 gestantes), incluíram gestantes primíparas e multíparas e não compararam o grupo de gestantes com um grupo de mulheres não gestantes. Além disso, em dois desses estudos,(6,22) apenas o 3º trimestre de gestação foi envolvido, e, em apenas dois estudos,(7,22) houve o acompanhamento das gestantes após o parto.
Ao se comparar as pressões respiratórias encontradas nesse estudo com os valores de referência para a população feminina brasileira na mesma faixa etária,(5) empregando-se a mesma metodologia, observaram-se valores mais baixos em ambos os grupos estudados.
De acordo com um estudo,(5) mulheres na faixa etária de 20-29 anos apresentariam uma média para PImáx e PEmáx de 101,6 e 114,1 cmH2O, respectivamente. Isso representaria para as gestantes um valor em torno de 87% do previsto, podendo ser interpretado como uma possível queda nos valores da força respiratória. No entanto, de acordo com a American Thoracic Society(25) e a European Respiratory Society,(26) valores de referência nos limites inferiores para os testes de função devem ser considerados abaixo do percentil 5% dos valores de referência, enquanto 80% do valor previsto não deve ser utilizado.
Apesar de a comparação acima não resultar em nenhuma repercussão clínica, essa levanta, no entanto, questionamentos quanto à uniformização dos valores de referência encontrados na literatura. As diretrizes respiratórias europeias,(26) americanas(25) e brasileiras(27) sobre função pulmonar alertam sobre a generalização de valores de referência para a população em geral, em virtude da grande variabilidade intersujeitos, e estimulam a construção de valores preditivos respeitando as diferenças socioeconômicas, antropométricas, geográficas e raciais para a aplicabilidade clínica.
O presente estudo considerou os vários fatores que podem interferir na mensuração de pressões respiratórias.(28) As mensurações partiram dos volumes pulmonares máximos, sempre precedidos de inspiração ou expiração rápida.(29) O orifício de fuga de 2 mm(30) e um clipe nasal foram utilizados. As gestantes foram incentivadas sempre da mesma maneira, com treinamento prévio e foram obtidas de três a cinco manobras tecnicamente aceitáveis, na mesma sessão de avaliação. O aprendizado de longo prazo foi minimizado, avaliando-se os grupos apenas uma vez. Além disso, a comparação dos valores das gestantes foi realizada entre uma população de nuligestas com o mesmo nível de atividade física e com semelhantes características sociais, antropométricas e raciais.
Dessa forma, diante do controle metodológico dos procedimentos, podemos considerar que os valores encontrados representam o comportamento da força muscular respiratória durante a gestação de mulheres na faixa etária de 20-29 anos na população aqui estudada. Por outro lado, embora os dados do presente trabalho tenham evidenciado que a força muscular respiratória não difere entre primigestas e nuligestas nessa faixa etária, tais resultados devem ser considerados com cautela, visto que o poder da amostra não foi suficiente para que os resultados sejam mais conclusivos. Por conseguinte, com base nos presentes dados, podemos sugerir que as pressões respiratórias em mulheres de 20-29 anos apresentam uma constância durante a gestação e são similares entre primigestas e nuligestas, embora não possa ser excluída a possibilidade de diferenças entre os grupos em relação à PImáx. Tais achados fornecem elementos para compreensão da
biomecânica muscular respiratória de mulheres no período gestacional.
Referências1. Black LF, Hyatt RE. Maximal respiratory pressures: normal values and relationship to age and sex. Am Rev Respir Dis. 1969;99(5):696-702.
2. Wilson SH, Cooke NT, Edwards RH, Spiro SG. Predicted normal values for maximal respiratory pressures in Caucasian adults and children. Thorax. 1984;39(7):535-8.
3. Vincken W, Ghezzo H, Cosio MG. Maximal static respiratory pressures in adults: normal values and their relationship to determinants of respiratory function. Bull Eur Physiopathol Respir. 1987;23(5):435-9.
4. Enright PL, Kronmal RA, Manolio TA, Schenker MB, Hyatt RE.
Respiratory muscle strength in the elderly. Correlates and reference values. Cardiovascular Health Study Research Group. Am J Respir Crit Care Med. 1994;149(2 Pt 1):430-8.
5. Neder JA, Andreoni S, Lerario MC, Nery LE. Reference values for lung function tests. II. Maximal respiratory pressures and voluntary ventilation. Braz J Med Biol Res. 1999;32(6):719-27.
6. Gilroy RJ, Mangura BT, Lavietes MH. Rib cage and abdominal volume displacements during breathing in pregnancy. Am Rev Respir Dis. 1988;137(3):668-72.
7. Contreras G, Gutiérrez M, Beroíza T, Fantín A, Oddó H, Villarroel L, et al. Ventilatory drive and respiratory muscle function in pregnancy. Am Rev Respir Dis. 1991;144(4):837-41.
8. García-Rio F, Pino JM, Gómez L, Alvarez-Sala R, Villasante C, Villamor J. Regulation of breathing and perception of dyspnea in healthy pregnant women. Chest. 1996;110(2):446-53.
9. Thomson KJ, Cohen ME. Studies in the circulation in pregnancy II. Vital capacity observations in normal pregnant women. Surg Gynecol Obstet. 1938;66:591-603.
10. Nørregaard O, Schultz P, Ostergaard A, Dahl R. Lung function and postural changes during pregnancy. Respir Med. 1989;83(6):467-70.
11. Khan ZA, Khan SA. Myotonic dystrophy and pregnancy. J Pak Med Assoc. 2009;59(10):717-9.
12. Argov Z, de Visser M. What we do not know about pregnancy in hereditary neuromuscular disorders. Neuromuscul Disord. 2009;19(10):675-9.
13. Atalah E, Castillo C, Castro R, Aldea A. Proposal of a new standard for the nutritional assessment of pregnant women [Article in Spanish]. Rev Med Chil. 1997;125(12):1429-36.
14. World Health Organization. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age. Methods and development. Geneva: World Health Organization, Department of Nutrition for Health and Development; 2006.
15. International Physical Activity Questionnaires - IPAQ [homepage on the Internet]. Huddinge: IPAQ [cited 2010 Aug 9]. Guidelines for data processing and analysis of the international physical activity questionnaire (IPAQ)- short and long forms. [Adobe Acrobat document, 15p.] Available from: http://www.ipaq.ki.se/scoring.pdf
16. Field SK, Bell SG, Cenaiko DF, Whitelaw WA. Relationship between inspiratory effort and breathlessness in pregnancy. J Appl Physiol. 1991;71(5):1897-902.
17. American Thoracic Society/European Respiratory Society. ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166(4):518-624.
18. Hautmann H, Hefele S, Schotten K, Huber RM. Maximal inspiratory mouth pressures (PIMAX) in healthy subjects--what is the lower limit of normal? Respir Med. 2000;94(7):689-93.
19. Marx GF, Murthy PK, Orkin LR. Static compliance before and after vaginal delivery. Br J Anaesth. 1970;42(12):1100-4.
20. McGinty AP. The comparative effects of pregnancy and phrenic nerve interruption on the diaphragm and their relation to pulmonary tuberculosis. Am J Obstet Gynecol. 1938;35:237-48.
21. Gee JB, Packer BS, Millen JE, Robin ED. Pulmonary mechanics during pregnancy. J Clin Invest. 1967;46(6):945-52.
22. Jensen D, Webb KA, Davies GA, O'Donnell DE. Mechanical ventilatory constraints during incremental cycle exercise in human pregnancy: implications for respiratory sensation. J Physiol. 2008;586(Pt 19):4735-50.
23. Gilleard WL, Brown JM. Structure and function of the abdominal muscles in primigravid subjects during pregnancy and the immediate postbirth period. Phys Ther. 1996;76(7):750-62.
24. De Deyne PG. Application of passive stretch and its implications for muscle fibers. Phys Ther. 2001;81(2):819-27.
25. Lung function testing: selection of reference values and interpretative strategies. American Thoracic Society. Am Rev Respir Dis. 1991;144(5):1202-18.
26. Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl. 1993;16:5-40.
27. Pereira CA. Espirometria. J Pneumol. 2002;28(Suppl 3):S1-S82.
28. Souza RB. Pressões respiratórias estáticas máximas. J Pneumol. 2002;28(Suppl 3):S155-165.
29. Zakynthinos S, Vassilakopoulos T, Mavrommatis A, Roussos C, Tzelepis GE. Effects of different expiratory maneuvers on inspiratory muscle force output. Am J Respir Crit Care Med. 1999;159(3):892-5.
30. Mayos M, Giner J, Casan P, Sanchis J. Measurement of maximal static respiratory pressures at the mouth with different air leaks. Chest. 1991;100(2):364-6.
* Trabalho realizado no Instituto de Medicina Integral Prof. Fernando Figueira, Recife (PE) Brasil
Endereço para correspondência: Andrea Lemos. Departamento de Fisioterapia, Avenida Professor Moraes Rego, 1235, Cidade Universitária, CEP 50670-901, Recife, PE, Brasil.
Tel 55 81 3328-6797. E-mail: lemosandrea@bol.com.br
Apoio financeiro: Nenhum.
Recebido para publicação em 9/8/2010. Aprovado, após revisão, em 11/1/2011.
Sobre os autoresAndrea Lemos
Professora. Departamento de Fisioterapia, Universidade Federal de Pernambuco, Recife (PE) Brasil.
Ariani Impieri Souza
Médica Obstétrica. Instituto de Medicina Integral Prof. Fernando Figueira, Recife (PE) Brasil.
Armele Dornelas de Andrade
Professora. Departamento de Fisioterapia, Universidade Federal de Pernambuco, Recife (PE) Brasil.
José Natal Figueiroa
Estatístico. Instituto de Medicina Integral Prof. Fernando Figueira, Recife (PE) Brasil.
José Eulálio Cabral-Filho
Professor. Instituto de Medicina Integral Prof. Fernando Figueira, Recife (PE) Brasil.







