Marina de Loureiro Maior, Renata Leborato Guerra, Michelle Cailleaux-Cezar, Jonathan Eric Golub, Marcus Barreto Conde
ABSTRACT
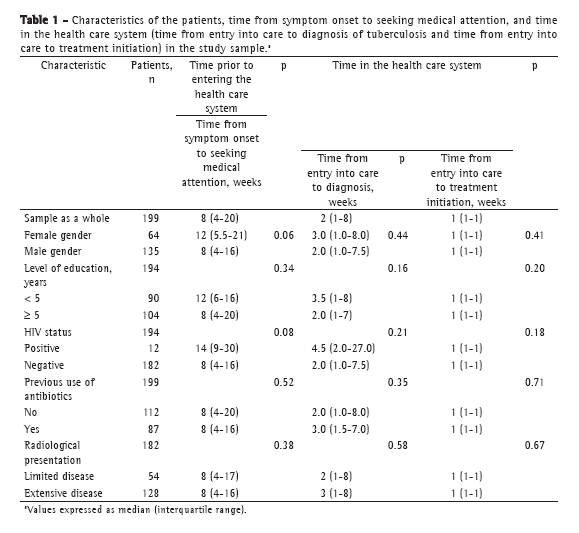
Objective: To estimate the time elapsed between the onset of symptoms and the initiation of treatment of pulmonary tuberculosis among treatment-naïve patients with positive results in sputum smear microscopy, and to evaluate the variables associated with delays in diagnosis and in treatment initiation. Methods: This was a descriptive exploratory study involving 199 treatment-naïve tuberculosis patients ≥ 12 years of age with AFB-positive sputum smear microscopy results between 2006 and 2008. At their first (treatment initiation) visit to a primary health care clinic in the city of Nova Iguaçu, Brazil, the patients were interviewed and their ancillary test results were reviewed. Results: The medians (and respective interquartile ranges) of the time from symptom onset to the initiation of treatment of pulmonary tuberculosis, from symptom onset to seeking medical attention, from entry into care to diagnosis, and from entry into care to treatment initiation, in weeks, were 11 (6-24), 8 (4-20), 2 (1-8), and 1 (1-1), respectively. The variables gender, age, level of education, previous use of antibiotics, HIV status, site of first medical visit, and radiological extent of tuberculosis showed no associations with the time from entry into care to diagnosis or to treatment initiation. The main reason for the delay in seeking medical attention reported by the patients was their inability to recognize their symptoms as indicators of a disease. Conclusions: Among the patients studied, there was an unacceptably long delay between the onset of symptoms and the initiation of tuberculosis treatment.
Keywords: Tuberculosis/diagnosis; Tuberculosis/therapy; Delayed diagnosis.
RESUMO
Objetivo: Estimar o tempo entre o início dos sintomas e o início do tratamento de pacientes com tuberculose pulmonar virgens de tratamento e com resultado positivo na baciloscopia direta do escarro, assim como avaliar as variáveis associadas à demora no diagnóstico e no início do tratamento. Métodos: Estudo descritivo exploratório em pacientes virgens de tratamento para tuberculose, com idade ≥ 12 anos e resultado positivo para BAAR no escarro. Entre 2006 e 2008, os 199 pacientes incluídos no estudo foram entrevistados, e seus exames complementares foram revisados no momento da consulta para o início de tratamento para tuberculose em uma unidade básica de saúde no município de Nova Iguaçu (RJ). Resultados: As medianas (e seus respectivos intervalos interquartílicos) para o tempo entre o início dos sintomas e o início do tratamento, o tempo até a procura por atendimento médico, o tempo até o diagnóstico e o tempo até o início do tratamento, em semanas, foram, respectivamente, 11 (6-24), 8 (4-20), 2 (1-8) e 1 (1-1).As variáveis gênero, idade, escolaridade, uso prévio de antibióticos, status HIV, local da primeira consulta médica e extensão radiológica da doença não se associaram ao tempo até o diagnóstico ou ao tempo até o início do tratamento. A principal razão para a demora dos pacientes em procurar o serviço de saúde foi sua dificuldade em reconhecer seus sintomas como indicativos de doença. Conclusões: Os tempos até o diagnóstico e até o início do tratamento para tuberculose foram inaceitavelmente longos na amostra estudada.
Palavras-chave: Tuberculose/diagnóstico; Tuberculose/terapia; Diagnóstico tardio.
Introduction