To the Editor:A female infant (age, 2 years and 4 months) from the city of São Paulo, Brazil, had been vaccinated with BCG in the maternity ward and presented with a 15-day history of pain in the right leg and impaired ambulation. The patient was treated with a nonsteroidal anti-inflammatory drug and showed improvement. However, two weeks after the drug had been discontinued, she showed worsening. An X-ray of the knee showed an osteolytic lesion in the right distal femoral epiphysis. Magnetic resonance imaging (Figure 1) revealed a centromedial lesion in the right distal femoral epiphysis, with multiple areas of cortical erosion; there was significant cortical discontinuity, and there was no effusion in the posteroinferior portion of the medial femoral condyle. The patient was treated with ceftriaxone for 14 days, with no improvement. A punch biopsy of the right knee showed a granuloma with no AFB. The patient was started on isoniazid, rifampin, and pyrazinamide. Investigation of her parents, siblings, and nannies showed no exposure to pulmonary or extrapulmonary tuberculosis. At admission to our facility, a few days after treatment initiation, the patient was in good general health. The only abnormality on physical examination was right knee edema (distal and proximal to the tibia). The edema was cold and painful on palpation, being accompanied by functional disability.
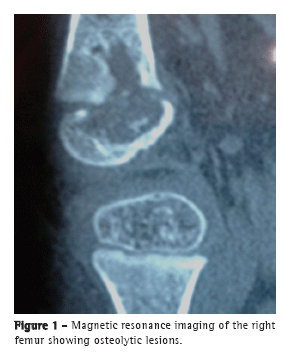
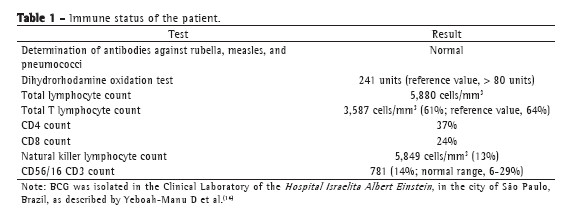
The following tests were performed: tuberculin skin testing with PPD, the induration being 14 mm; X-ray and magnetic resonance imaging of the right knee; enzyme-linked immunospot (ELISPOT) assay, the results being negative; knee biopsy, revealing very little bone tissue with two epithelioid granulomas (one of which had caseous necrosis) and chronic lymphoplasmacytic inflammatory infiltrate; AFB testing, the results being negative; mycobacterial culture, the results being positive; PCR testing, revealing the presence of insertion sequence 6110 (which is characteristic of mycobacteria) and duplication of spacer 33 in the DR region (172-bp amplicon), present only in the Mycobacterium bovis BCG strain and absent in M. tuberculosis; PCR testing, the results being negative for M. tuberculosis; routine tests (complete blood count, ESR determination, HIV testing, and evaluation of liver and kidney function), the results being normal; humoral and cellular immune response testing, the results being normal (Table 1); and chest CT, the findings being normal.
The patient achieved a satisfactory clinical improvement and was discharged after 18 months of treatment, at which point an X-ray of the right femur showed normal findings.
The BCG vaccine is used in many countries, and there are no strict rules regarding the age of vaccination, the groups that should be vaccinated, the type of vaccine, the concentration of bacilli, the ratio of live to dead bacilli, or the mode of administration. Many strains are used; however, there is uncertainty regarding the total number of viable and nonviable bacilli (which could potentiate the immunity induced by the former) and the ability of PPD to induce allergy, both of which are factors that can have an impact on the potency and complications of the vaccine.(1,2) In Brazil, vaccination with BCG Moreau is given intradermally (at the insertion of the right deltoid) in the first days of life, at concentrations of 300,000-1,000,000 bacilli/dose.
Severe side effects are rare and include persistent ulceration at the vaccination site, surrounding adenopathy, osteitis, and disseminated disease, which can occur in severely immunocompromised individuals (including the cases of locally administered vaccine in patients with bladder carcinoma). In the Czech Republic, the incidence of complications has decreased with the use of lower doses of the vaccine.(3)
In Russia, the incidence of bone tuberculosis has been reported to be higher than that of primary tuberculosis, BCG having been confirmed as the cause of osteitis in 46% of the patients who presented with the disease.(4)
In Japan, the incidence of osteitis has been reported to be 0.2 cases/100,000 vaccinations, varying according to the BCG strain(5) and the use of vaccines that are less reactogenic.(6) In Finland, the incidence of osteitis has also been reported to vary according to the BCG strain.(7)
We found no reports of osteitis caused by BCG Moreau. However, it should be noted that BCG Moreau is not as widely used as are other strains, particularly BCG Glaxo and BCG Pasteur-Paris. In Brazil, one group of authors(8) recently reported the case of a normal-weight, immunocompetent child who presented with radial and ulnar lesions and who was highly suspected of having BCG osteitis, although the bacillus was not identified.(8)
The diagnosis of BCG osteitis in our patient is indisputable. Tuberculin skin testing confirmed that PPD positivity does not depend on M. tuberculosis, given that ELISPOT assay results were negative. Biopsy showed a caseating granuloma, no bacilli having been identified. However, culture was positive, confirming mycobacteriosis. The presence of BCG was confirmed by PCR revealing insertion sequence IS6110, which is characteristic of the vaccine. Therefore, this seems to be the first confirmed case of osteitis after vaccination with BCG Moreau. The classic criteria of Foucard & Hjelmstedt(9) are useful in raising the suspicion of BCG osteitis, because they include vaccination in the neonatal period, development of symptomatic disease up to four years after vaccination, no contact with tuberculosis, a clinical profile consistent with tuberculosis, and histopathological findings consistent with tuberculosis. Our patient met those criteria. However, the current gold standard is the detection of the presence of M. bovis.
The patient was treated with isoniazid, rifampin, and pyrazinamide because of the presumptive diagnosis of tuberculosis. The hypothesis of osteitis after BCG vaccination was raised later, pyrazinamide being discontinued because BCG is resistant to the drug. The treatment continued for 18 months, and complete cure was achieved. However, treatment with only isoniazid and rifampin for 6 months has been reported to be successful. Recurrence is rare.
Osteitis after BCG vaccination is slightly more common in boys than in girls. Humeral osteitis results from contiguous spread, which was impossible in our patient. Osteitis resulting from hematogenous spread is more likely, occurring primarily in the epiphyses of long bones in the arms and legs (which are highly vascularized) and being usually solitary.(5,10) Involvement of other bones, such as the sternum(11) and the sacrum,(12) is extremely rare. The unilaterality of the disease suggests that circulation was increased (by minimal trauma, for instance) prior to the onset of the bacilli. However, it is strange that other highly vascularized organs are more resistant.
Dissemination of BCG is facilitated by immunosuppression, which was not observed in our patient. She had a normal complete blood count and normal counts of lymphocytes, CD4, CD8, and natural killer lymphocytes, as well as having normal levels of antibodies against rubella, measles, and pneumococci. In addition, she had a normal dihydrorhodamine oxidation test result. Unfortunately, it was impossible to determine IL-12 levels, and deficiency/absence of IL-12 might be related to mycobacterial disease. In Brazil, this condition was found in two brothers with BCG adenitis and deficient IL-12 production after stimulation with INF-γ.(13)
Nelson Morrone
Collaborator,
Clemente Ferreira Institute,
São Paulo State Department of Health, São Paulo, Brazil
Cláudio do Amaral Antonio
Physician,
Clemente Ferreira Institute,
São Paulo State Department of Health, São Paulo, Brazil
Cláudio Santilli
Physician,
Hospital Israelita Albert Einstein,
São Paulo, Brazil
Beatriz Tavares Costa Carvalho
Adjunct Professor,
Division of Allergy and Immunology and Division of Rheumatology, Department of Pediatrics,
Federal University of São Paulo,
São Paulo, Brazil
Denise Rodrigues
Physician,
Clemente Ferreira Institute,
São Paulo State Department of Health, São Paulo, BrazilReferences1. World Health Organization [homepage on the Internet]. Geneva: World Health Organization. [cited 2011 Sep 9]. Fine PE, Carneiro IA, Milstien JB, Clements CJ. Issues relating to the use of BCG in immunization programmes. A discussion document [Adobe Acrobat document, 45p.]. Available from: http://www.who.int/vaccines-documents/DocsPDF99/www9943.pdf
2. Milstien JB, Gibson JJ. Quality control of BCG vaccine by WHO: a review of factors that may influence vaccine effectiveness and safety. Bull World Health Organ. 1990;68(1):93-108. PMid:2393003.
3. Vítková E, Galliová J, Krepela K, Kubín M. Adverse reactions to BCG. Cent Eur J Public Health. 1995;3(3):138-41. PMid:8535371.
4. Kamaeva NG, Chugaev IuP, Grinberg LM, Anisimova NA, Golubeva TV, Kamaev EIu. Clinical and epidemiological features of tuberculosis ostitis in BCG-vaccinated children [Article in Russian]. Probl Tuberk Bolezn Legk. 2009;(1):16-20. PMid:19253678.
5. Koyama A, Toida I, Nakata S. Osteitis as a complication of BCG vaccination [Article in Japanese]. Kekkaku. 2009;84(3):125-32. PMid:19364044.
6. Jou R, Huang WL, Su WJ. Tokyo-172 BCG vaccination complications, Taiwan. Emerg Infect Dis. 2009;15(9):1525-6.
7. Kröger L, Korppi M, Brander E, Kröger H, Wasz-Höckert O, Backman A, et al. Osteitis caused by bacille Calmette-Guérin vaccination: a retrospective analysis of 222 cases. J Infect Dis. 1995;172(2):574-6. PMid:7622909.
8. Yamada AF, Pellegrini JB, Cunha LM, Fernandes Ada R. Osteitis after BCG vaccination. J Bras Pneumol. 2009;35(3):285-9. PMid:19390729. http://dx.doi.org/10.1590/S1806-37132009000300015
9. Foucard T, Hjelmstedt A. BCG-osteomyelitis and -osteoarthritis as a complication following BCG-vaccination. Acta Orthop Scand. 1971;42(2):142-51. PMid:4939582.
10. Marík I, Kubát R, Filipský J, Galliová J. Osteitis caused by BCG vaccination. J Pediatr Orthop. 1988;8(3):333-7. PMid:3284907.
11. Saifudheen K, Anoop TM, Mini PN, Ramachandran M, Jabbar PK, Jayaprakash R. Primary tubercular osteomyelitis of the sternum. Int J Infect Dis. 2010;14(2):e164-6. PMid:19524467.
12. Meurice JC, Dore P, Lamotte F, Bataille B, Levard G, Castets M, et al. Sacral tuberculous osteitis [Article in French]. Rev Mal Respir. 1994;11(4):418-20.
13. Lawrence TC, Carvalho BC. Adenite pós vacina BCG em dois pacientes com imunodeficiência de receptor 1 do INFgama. Rev Bras Alerg Imunopatol. 2006;29(1):18-23.
14. Yeboah-Manu D, Yates MD, Wilson SM. Application of a simple multiplex PCR to aid in routine work of the mycobacterium reference laboratory. J Clin Microbiol. 2001;39(11):4166-8. http://dx.doi.org/10.1128/JCM.39.11.4166-4168.2001



