ABSTRACT
Objective: To retrospectively analyze the medical charts of patients with pulmonary malformations submitted to surgical treatment and
to investigate the clinical evolution prior to the definitive diagnosis. Methods: We analyzed the medical charts of patients with pulmonary
malformations operated on at the São Paulo Hospital-Federal University of São Paulo/Paulista School of Medicine-from 1969 to 2004.
Each medical chart was analyzed as to the following aspects: clinical profile; diagnosis; previous treatment; surgical treatment; and
nosocomial complications. The inclusion criteria were having received a diagnosis of pulmonary malformation, having undergone pulmonary
resection, and chart data being complete. Results: The analysis of the medical charts revealed that 60 patients diagnosed with pulmonary
malformations-27 cases of bronchogenic cyst, 14 cases of congenital lobar emphysema, 10 cases of pulmonary sequestration, and 9 cases of
cystic adenomatoid malformation-underwent surgery. Ages ranged from 4 days to 62 years (mean, 17.9 years). There was a predominance of
males (55%). Ninety-two percent of the patients presented symptoms (mean duration, 15.37 months). Of the 60 patients undergoing surgery,
27 (45%) received preoperative home or hospital treatment with antibiotics. Regarding complications, we observed that morbidity was 23%,
and mortality was 3.3%. Surgical times ranged from 1 to 8 h (mean, 3.2 h). Conclusions: Misdiagnosis or delayed diagnosis of pulmonary
malformations resulted in unnecessary treatments and hospitalizations, as well as in frequent, recurrent infectious complications. We believe
that the definitive treatment is surgery, which is curative and has low morbidity and mortality rates.
Keywords:
Bronchogenic cyst; Bronchopulmonary sequestration; Cystic adenomatoid malformation of lung, congenital; Respiratory tract infections; Thoracic surgery.
RESUMO
Objetivo: Analisar retrospectivamente os prontuários de pacientes com malformações pulmonares submetidos a tratamento operatório e
verificar a evolução clínica até o diagnóstico definitivo. Métodos: Analisamos os prontuários dos pacientes com malformações pulmonares
operados no Hospital São Paulo-Universidade Federal de São Paulo/Escola Paulista de Medicina-de 1969 a 2004. Cada prontuário foi analisado
quanto aos seguintes aspectos: quadro clínico, diagnóstico, tratamento prévio, tratamento operatório e complicações hospitalares. Os
critérios de inclusão foram os seguintes: ter diagnóstico de malformação pulmonar, ter sido submetido à ressecção pulmonar e ter prontuário
com dados completos. Resultados: A análise dos prontuários revelou que 60 pacientes com diagnóstico de malformações pulmonares foram
operados-27 casos de cisto broncogênico, 14 de ensifema lobar congênito, 10 de seqüestro pulmonar e 9 de malformação adenomatóide
cística. A idade variou de 4 dias a 62 anos (média de 17,9 anos). Houve predominância do sexo masculino (55%). Noventa e dois por cento
dos pacientes apresentavam sintomas (média de duração, 15,37 meses). Dos 60 pacientes operados, 27 (45%) receberam tratamento domiciliar
ou hospitalar com antibiótico antes da operação. Quanto às complicações, observamos morbidade de 23% e mortalidade de 3,3%. A
duração dos procedimentos operatórios realizados em nossos pacientes variou de 1 a 8 h (média, 3,2 h). Conclusões: A falha ou atraso no
diagnóstico das malformações pulmonares resultou em tratamentos e hospitalizações desnecessárias e em complicações infecciosas recorrentes
e freqüentes. Acreditamos que o tratamento definitivo é a operação, a qual é curativa e tem baixa morbidade e mortalidade.
Palavras-chave:
Cisto broncogênico; Seqüestro broncopulmonar; Malformação adenomatóide cística congênita do pulmão;Infecções respiratórias; Cirurgia torácica.
IntroduçãoAs cavidades aéreas no pulmão foram descritas pela primeira vez por Fontanus em 1638; já as doenças císticas pulmonares congênitas foram descritas em 1687 por Bartholinus e Marcellus Malpighius no livro "Opera Omnia". As lesões congênitas torácicas são raras e de expressão clínica ainda incerta.(1)
Está claro que a maior parte da morfogênese alveolar ocorre no período pós-natal. Estudos morfométricos estimam que somente 8% (entre 20 e 50 milhões) dos alvéolos da vida adulta estão presentes ao nascimento. Após os 8 anos de idade, as unidades alveolares continuam crescendo e se desenvolvendo, porém de forma mais lenta, caracterizada pelo aumento do tamanho dos alvéolos (hipertrofia), chamado de crescimento pulmonar compensatório. Aos 9 anos, existem aproximadamente 280 milhões de alvéolos, número próximo ao encontrado na vida adulta.(2,3)
A incidência das malformações pulmonares varia de 30 a 42 casos para cada 100.000 habitantes por ano-de 0,06 a 2,2% dos pacientes internados em hospitais gerais.(4,5) Suas formas de apresentação são variáveis, podendo ser muito graves, até com morte ao nascer, ou totalmente assintomáticas até a vida adulta. É importante reconhecer essas afecções para que o tratamento adequado seja instituído (na maioria das vezes, operatório), evitando-se, assim, tratamentos desnecessários.
O objetivo deste estudo foi realizar uma análise retrospectiva dos prontuários de pacientes com malformações pulmonares submetidos a tratamento operatório e verificar a evolução clínica até a terapêutica definitiva.
MétodosAnalisamos retrospectivamente os prontuários dos pacientes com malformações pulmonares operados no Hospital São Paulo-Universidade Federal de São Paulo/Escola Paulista de Medicina-de 1969 a 2004. Os seguintes critérios de inclusão foram adotados: ter diagnóstico de malformação pulmonar (confirmado por exame anatomopatológico), ter sido submetido à ressecção pulmonar e ter prontuário com dados completos.
Cada prontuário foi analisado de forma sistemática quanto aos seguintes aspectos: quadro clínico, diagnóstico, tratamento prévio, tratamento operatório e complicações hospitalares. Os itens avaliados foram divididos em dois tipos: dados clínicos pré-operatórios (idade no momento da operação, sexo, infecção prévia ao tratamento definitivo, e presença e duração dos sintomas) e dados pós-operatórios (extubação na sala operatória, necessidade de cuidados em unidade de terapia intensiva (UTI) no período pós-operatório, duração dos procedimentos, tipo de procedimento realizado, tempo de internação e complicações).
Este trabalho foi aprovado pelo Comitê de Ética e Pesquisa da instituição (CEP 0496/04).
ResultadosA análise dos prontuários revelou que 60 pacientes com diagnóstico de malformações pulmonares foram operados-27 casos de cisto broncogênico, 14 de enfisema lobar congênito, 10 de seqüestro pulmonar e 9 de malformação adenomatóide cística.
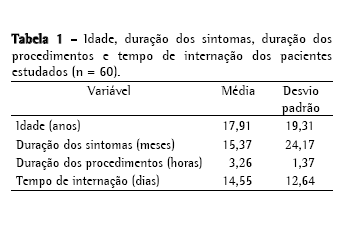
Dados clínicos pré-operatóriosA idade variou de 4 dias a 62 anos, com média de 17,9 anos (Tabela 1). A maior incidência ocorreu no primeiro ano de vida (28,3% dos pacientes). Do total, 32 (53,3%) dos pacientes foram operados com idade inferior a 13 anos (Figura 1).
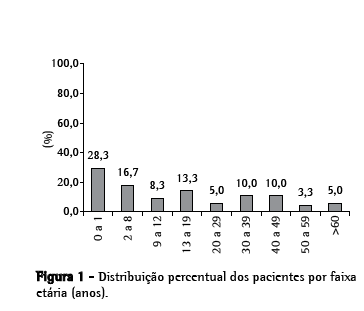
Quanto à distribuição por sexo, prevaleceu o masculino-33 (55%) vs. 27 (45%).
Pudemos constatar que a maioria (92%) dos pacientes apresentava sintomas, sendo estes pouco específicos, como tosse, dispnéia ou desconforto torácico, mas que foram motivo suficiente para que os pacientes procurassem atendimento médico (Tabela 2). A duração dos sintomas também foi avaliada.
Apesar da inespecificidade das queixas, a persistência chegou a até 36 meses em 3 pacientes, com média de 15,37 meses (Tabela 1).
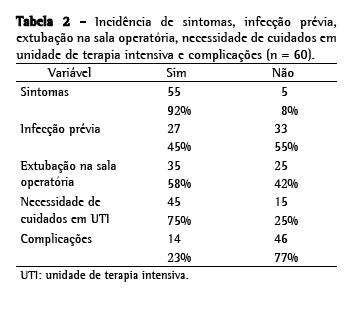
O tratamento domiciliar ou hospitalar com antibióticos antes da operação foi realizado em 27 (45%) dos pacientes (Tabela 2). A recorrência das infecções pulmonares (pneumonia de repetição) esteve presente em 18 (30%) dos pacientes. Em 7 (11,7%) dos pacientes, foram relatados mais de cinco tratamentos, e em 11 (18,3%), de dois a quatro tratamentos. Em 1 (1,7%) dos pacientes, o tratamento com antibiótico foi relatado dez vezes, nove com internações hospitalares, sem o diagnóstico de malformação pulmonar.
Dados pós-operatóriosA extubação ao final do procedimento, ainda na sala operatória, foi verificada em 58% dos pacientes. A recuperação no período pós-operatório imediato foi realizada na UTI em 75% dos pacientes (Tabela 2). A duração dos procedimentos operatórios variou de 1 a 8 h, com média de 3,2 h (Tabela 1).
Os procedimentos realizados foram: lobectomia pulmonar (58%), cistectomia (13%), segmentectomia pulmonar (13%), sequestrectomia (10%), bilobectomia (3%) e pneumonectomia (3%). O tempo de internação variou de 3 a 70 dias, com média de 14,55 dias (Tabela 1).
Quanto às complicações, observamos uma morbidade de 23% (14 pacientes, sendo que 4 tiveram mais de uma complicação). Os tipos de complicações foram pneumonia (6 casos), atelectasia (6 casos), empiema pleural (3 casos), sepse (2 casos) e óbito (2 casos). Também tivemos 1 paciente com coágulo retido e necessidade de reoperação. A mortalidade foi de 3,3%-2 pacientes com malformações operados antes de 1 ano de idade, em períodos distintos, e que evoluíram com atelectasia, pneumonia, sepse e óbito (um deles inclusive com um tempo de internação de 70 dias).
DiscussãoAs malformações pulmonares são conhecidas pelos médicos há mais de três séculos, e ainda não há consenso de como e quando tratar esses pacientes. Ainda que consideremos a incidência pequena, ela pode ser até duas vezes maior que a incidência de neoplasia pulmonar no estado de São Paulo-17,6 pacientes para cada 100.000 habitantes.(6)
As alterações que levam às malformações ainda não são totalmente conhecidas. Sabemos que podem ocorrer da segunda até a décima sexta semana de gestação, porém ainda há controvérsias na literatura.(1,5,7)
Quando nos lembramos de doenças pulmonares congênitas, as associamos a pacientes assintomáticos, mas, ao revisarmos a literatura, verificamos que isso não corresponde à realidade. De fato, as malformações apresentam sintomas na maioria dos pacientes.(1,8-12) Em uma pequena porcentagem deles, a doença será descoberta em exames de imagem realizados de forma rotineira ou ainda por outra indicação não-respiratória. Os pacientes com malformações pulmonares apresentarão sintomas em algum momento da vida, a maioria antes dos 13 anos de idade. Nesta série, 92% dos pacientes eram sintomáticos na ocasião do diagnóstico. Nossos resultados são semelhantes aos de outras séries da literatura, nas quais se relata que a maioria desses pacientes (de 65 a 100%), com idade inferior a 15 anos, são sintomáticos.(8-12) A conduta em pacientes assintomáticos é controversa; porém, em razão do potencial risco de complicações, essas lesões são cada vez mais ressecadas precocemente.(13,14) Mesmo em pacientes assintomáticos, há o risco de eventuais complicações e a infecção respiratória é a mais comum.
O prognóstico dessa afecção é imprevisível, sendo relatado que 45% dos pacientes com malformações pulmonares teriam algum tipo de complicação antes do tratamento definitivo. Essas complicações incluiriam hemoptise, pneumotórax, compressão esofágica, pleurite, infecções dos cistos e pneumonia.(15,16) Outras complicações que podem ocorrer são o desenvolvimento de lesões malignas (carcinomas ou sarcomas pulmonares), pneumotórax, hemoptise e hemotórax. Como mais cedo ou mais tarde a ressecção pulmonar será necessária, o melhor é agir antes que tais complicações ocorram.(13,17) Houve, nesta série, 1 paciente do sexo feminino com adenocarcinoma na parede do cisto congênito ressecado.
Em nossa série, houve necessidade de tratamento domiciliar ou hospitalar conseqüente a infecções pulmonares prévias ou a diagnósticos errôneos em 27 (45%) dos pacientes. As infecções foram caracterizadas como pneumonia, sendo que tivemos 1 paciente com nove hospitalizações anteriores ao diagnóstico de malformação. Um autor mostrou atrasos de até 7 anos no diagnóstico, com média de 1,6 ano; os pacientes permaneceram sintomáticos nesse período, e todas as radiografias de tórax eram alteradas.(18) Uma revisão envolvendo 320 crianças com diagnóstico de pneumonia enfatizou a correlação clínico-radiográfica com a avaliação de três profissionais-um radiologista pediátrico, um pneumologista pediátrico e um pediatra de pronto-socorro. A concordância entre os observadores foi de somente 41%.(19) Em nosso meio, existe uma banalização evidente do diagnóstico de pneumonia, em particular do chamado "começo de pneumonia". Muitos desse pacientes que apresentam tosse, coriza e febre, bem como uma radiografia de tórax alterada, são (ou foram) tratados como portadores de pneumonia. Entretanto, a imagem do exame poderia corresponder a uma malformação, como o cisto broncogênico, e o quadro clínico, ao de uma infecção de via aérea superior.
O diagnóstico errôneo mais freqüente em pacientes com malformações pulmonares é a pneumonia, e o diagnóstico correto no período pré-operatório é feito em apenas 30% dos pacientes.(8) A falha no diagnóstico das malformações resulta em tratamentos desnecessários, complicações infecciosas recorrentes e hospitalizações freqüentes. Mesmo com o atraso no diagnóstico, todos os pacientes podem ser curados com o tratamento operatório. Acreditamos que o tratamento operatório não deve ser realizado na fase aguda da infecção respiratória, exceto em situações específicas, com falha da terapêutica clínica.(1)
O tratamento operatório é indicado em razão do risco de infecção, interferência no crescimento pulmonar (mesmo lado ou contralateral), insuficiência cardíaca e malignização das lesões pulmonares. Durante a gestação, podemos optar por observação clínica ou por intervenções pré-natais nos fetos com derrames pleurais ou cistos hiperinsuflados, tratados, na maioria das vezes, com toracocentese. Se necessário, pode-se recorrer à lobectomia intra-uterina. No período pós-natal, o tratamento mais indicado é a ressecção da malformação, geralmente com prognóstico excelente.(1,11,20,21)
Quanto maior o número de infecções pulmonares antes do tratamento operatório, maior a dificuldade do ato operatório em si em razão da presença de inflamação ao redor da malformação. Há maior probabilidade de encontrarmos aderências pleurais e tecido fibrótico ao redor da lesão, e, com isso, o tempo operatório pode ser mais prolongado. Essa probabilidade é menor em pacientes assintomáticos. A justificativa para essa diferença no tempo operatório seria a maior duração da doença, com mais infecções e inflamações na própria lesão e/ou no parênquima adjacente. Com mais inflamações e aderências, a ressecção tende a ser mais lenta e cautelosa; com isso, o tempo operatório é maior. Na literatura encontramos alguns relatos sobre a dificuldade durante o ato operatório em pacientes sintomáticos no período pré-operatório.(10,16,22) A freqüência de dificuldades durante a operação é maior em pacientes sintomáticos do que em pacientes assintomáticos; portanto, o tratamento operatório deve ser realizado, de preferência, enquanto os pacientes ainda são assintomáticos.(1,23)
O tratamento operatório é recomendado, e há ainda a recomendação de que a lobectomia deve ser a operação padrão.(23) Atualmente prefere-se a lobectomia somente para as malformações que acometem todo o lobo.
Sempre que possível e seguro, resseca-se somente a área pulmonar malformada, seja por uma segmentectomia anatômica ou não-anatômica, conforme já relatado na literatura.(24,25) Apesar disso, a lobectomia ainda é o tipo de ressecção mais utilizada para o tratamento das malformações pulmonares, inclusive nesta série.(9,26-28)
A extubação ao final do procedimento na sala operatória foi realizada em 58% dos nossos pacientes. A necessidade de cuidados em UTI no período pós‑operatório imediato ocorreu em 75% dos pacientes. As crianças exigiram um zelo maior no período pós-operatório, talvez mais por precaução do que por real necessidade. Para os pacientes com esse tipo de lesão pulmonar congênita, geralmente jovem e sem outras co-morbidades, se o procedimento não tiver intercorrências, a recuperação anestésica já seria suficiente no período pós-operatório imediato.(13,14,28)
Temos uma preocupação constante com o tempo de internação, e nossa média de internação foi de 14,55 dias. Cumpre ressaltar que este estudo retrospectivo foi longo e sujeito a falhas. Durante as três décadas de revisão, a medicina passou por muitas mudanças e, atualmente, a investigação diagnóstica é realizada preferencialmente no ambulatório, diminuindo o tempo necessário de internação.
As complicações ocorreram em 23% dos pacientes, com uma mortalidade de 3,3%. Dentre as complicações, prevaleceu a atelectasia (5 crianças e 1 adulto), principalmente nos pacientes abaixo de 2 anos, o que se justifica pelo diminuto calibre das vias aéreas frente a secreções, por vezes tão espessas como nos adultos. Quando dividimos os pacientes por faixa etária, verificamos uma maior morbidade e mortalidade nas crianças abaixo de 1 ano (atelectasia e pneumonia em 58% e óbito em 11,8% dos pacientes). Cabe ressaltar que, em pacientes nessa faixa etária, devem ser ponderadas as indicações para a operação sempre que o quadro clínico permitir. Se conseguirmos postergar o tratamento operatório para próximo de 1 ano de vida ou mais, as taxas de morbidade e de mortalidade serão menores. Na literatura encontramos incidências semelhantes, com morbidade de 40% e mortalidade ao redor de 10%. Dentre as complicações mais freqüentes relatadas temos drenagem prolongada, empiema, pneumonia e infecção da ferida operatória.(11,16,29) Nesta série tivemos taxas de morbidade e de mortalidade bem aquém das relatadas na literatura. Cabe lembrar que as complicações que podem ocorrer antes do tratamento operatório, causadas pela própria malformação, atingem até 45% dos pacientes,(16) incidência maior do que a morbidade no período pós-operatório.
Não existe consenso na literatura mundial sobre qual seria a idade ideal para se realizar o tratamento operatório após o nascimento. Há justificativas para a operação tanto no paciente adulto quanto na criança. Porém, sabemos que o crescimento pulmonar por hiperplasia (aumento do número de alvéolos de fato) ocorre até os 8 anos de idade. Após os 9 anos de idade há o crescimento pulmonar em volume e tamanho, mas não no número absoluto de alvéolos.(1) Em 1987, 230 pacientes submetidos à pneumonectomia foram acompanhados por até 30 anos. Na época da operação, os pacientes foram divididos por faixa etária. O grupo operado com idade entre 0 e 5 anos manteve a capacidade pulmonar total em 96% mesmo após a pneumonectomia.(30) Outros autores recomendam que o tratamento operatório de pacientes com diagnóstico de malformações pulmonares seja realizado entre 3 e 6 meses de vida para que possa ocorrer o crescimento pulmonar.(13,29,30)
Concluímos que as malformações pulmonares foram subdiagnosticadas em nosso serviço. Por vezes, diversas situações foram confundidas com infecções pulmonares e, por vezes, tratadas como pneumonia. A hipótese diagnóstica de alteração pulmonar congênita deve ser, pelo menos, considerada pelo médico, principalmente em pacientes com pneumonia recorrente. Reiteramos que a falha ou o atraso no diagnóstico das malformações pulmonares resultou em tratamentos e hospitalizações desnecessárias, assim como em complicações infecciosas recorrentes e freqüentes. Acreditamos que o tratamento definitivo é a operação. Há uma tendência à menor ressecção possível e segura, sendo que, atualmente, reservamos a indicação de lobectomia para os pacientes que têm todo o lobo comprometido. Em pacientes ainda assintomáticos, mas com evidências de malformação pulmonar em exames de imagem, a operação deve ser indicada mesmo sem o diagnóstico confirmado. Temos como preferência, se possível, realizar o tratamento próximo ou acima da idade de 1 ano. Ressaltamos que o tratamento cirúrgico é curativo, com baixa morbidade e mortalidade em nosso serviço.
Outros trabalhos são necessários para a elucidação de controvérsias e, principalmente, para o esclarecimento da população médica em geral quanto à importância do diagnóstico correto dessas doenças.
Referências1. Costa Jr AS. Análise retrospectiva do tratamento operatório das malformações torácicas (pulmonares e linfáticas). [dissertation]. São Paulo: Universidade Federal de São Paulo; 2006.
2. Ruiz Jr RL, Carvalho LR, Catâneo AJ. Crescimento pulmonar compensatório (CPC): massa corpórea, conteúdo protéico e massa pulmonar em ratos subnutridos trilobectomizados. Acta Cir Bras. 2004;19(2):146-52.
3. Catâneo AJM, Catâneo DC. Crescimento pulmonar compensatório em implante lobar autólogo pós-pneumonectomia em cães. Acta Cir Bras. 2005;20(5):368-74.
4. Juan ES. Tratamento dos cistos congênitos do pulmão. [thesis for tenure]. São Paulo: Universidade de São Paulo; 1954.
5. Skandalakis JE, Gray SW, Symbas P. The trachea and the lungs. In: J.E. Skandalakis JE, Gray SW, editors. Embryology for surgeons: the embryological basis for the treatment of congenital anomalies. 2nd ed. Baltimore: Williams & Wilkins; 1994. p. 414-450.
6. Zamboni M. Epidemiologia do cancêr do pulmão. J Pneumol. 2002;28(1):41-7.
7. Landing BH. Five syndromes (malformation complexes) of pulmonary symmetry, congenital heart disease, and multiple spleens. Pediatr Pathol. 1984;2(2):148-51.
8. Ramenofsky ML, Leape LL, McCauley RG. Bronchogenic cyst. J Pediatr Surg. 1979;14(3):219-24.
9. Snyder ME, Luck SR, Hernandez R, Sherman JO, Raffensperger JG. Diagnostic dilemmas of mediastinal cysts. J Pediatr Surg. 1985;20(6):810-5.
10. St-Georges R, Deslauriers J, Duranceau A, Vaillancourt R, Deschamps C, Beauchamp G, et al. Clinical spectrum of bronchogenic cysts of the mediastinum and lung in the adult. Ann Thorac Surg. 1991;52(1):6-13.
11. Nobuhara KK, Gorski YC, La Quaglia MP, Shamberger RC. Bronchogenic cysts and esophageal duplications: common origins and treatment. J Pediatr Surg. 1997;32(10):1408-13.
12. Louie HW, Martin SM, Mulder DG. Pulmonary sequestration: 17-year experience at UCLA. Am Surg. 1993;59(12):801-5.
13. Laberge JM, Puligandla P, Flageole H. Asymptomatic congenital lung malformations. Semin Pediatr Surg. 2005;14(1):16-33.
14. Shanmugam G. Adult congenital lung disease. Eur J Cardiothorac Surg. 2005;28(3):483-9.
15. Brown RK, Robbins LL. Diagnosis and treatment of bronchogenic cysts of the mediastinum and lung. J Thorac Surg. 1944;13(1):84-105.
16. Sarper A, Ayten A, Golbasi I, Demircan A, Isin E. Bronchogenic cyst. Tex Heart Inst J. 2003;30(2):105-8.
17. Endo C, Imai T, Nakagawa H, Ebina A, Kaimori M. Bronchioloalveolar carcinoma arising in a bronchogenic cyst. Ann Thorac Surg. 2000;69(3):933-5.
18. Gustafson RA, Murray GF, Warden HE, Hill RC, Rozar GE. Intralobar sequestration. A missed diagnosis. Ann Thorac Surg. 1989;47(6):841-7.
19. Sarria E, Lima JB, Fischer GB, Barreto SS, Flôres JA, Sukiennik R. Concordância no diagnóstico radiológico das infecções respiratórias agudas baixas em crianças. J Pediatr (Rio J). 2003;79(6):497-503.
20. Canals-Riazuelo J, Boix Ochoa J, Peiro JL, Ezzedine M, Cobos Barroso N, Liñan Cortés S, et al. [Congenital lobar emphysema: report of 38 cases][Article in Spanish]. Cir Pediatr. 1994;7(2):97-101.
21. Conran RM, Stocker JT. Extralobar sequestration with frequently associated congenital cystic adenomatoid malformation, type 2: report of 50 cases. Pediatr Dev Pathol. 1999;2(5):454-63.
22. Aktoğu S, Yuncu G, Halilçolar H, Ermete S, Buduneli T. Bronchogenic cysts: clinicopathological presentation and treatment. Eur Respir J. 1996;9(10):2017-21.
23. Ribet ME, Copin MC, Gosselin BH. Bronchogenic cysts of the lung. Ann Thorac Surg. 1996;61(6):1636-40.
24. Browdie D, Todd D, Agnew R, Rosen W, Beardmore H. The use of "nonanatomic" pulmonary resection in infants with extensive congenital adenomatoid malformation of the lung. J Thorac Cardiovasc Surg. 1993;105(4):732-6.
25. van Koningsbruggen S, Ahrens F, Brockmann M, Michalk D, Rietschel E. Congenital cystic adenomatoid malformation type 4. Pediatr Pulmonol. 2001;32(6):471-5.
26. Leape LL, Longino LA. Infantile lobar emphysema. Pediatrics. 1964;34:246-55.
27. Murray GF. Congenital lobar emphysema. Surg Gynecol Obstet. 1967;124(3):611-25.
28. Ozçelik U, Göçmen A, Kiper N, Doğru D, Dilber E, Yalçin EG. Congenital lobar emphysema: evaluation and long-term follow-up of thirty cases at a single center. Pediatr Pulmonol. 2003;35(5):384-91.
29. Kim YT, Kim JS, Park JD, Kang CH, Sung SW, Kim JH. Treatment of congenital cystic adenomatoid malformation-does resection in the early postnatal period increase surgical risk? Eur J Cardiothorac Surg. 2005;27(4):658-61.
30. Laros CD, Westermann CJ. Dilatation, compensatory growth, or both after pneumonectomy during childhood and adolescence. A thirty-year follow-up study. J Thorac Cardiovasc Surg. 1987;93(4):570-6.
Trabalho realizado na Disciplina de Cirurgia Torácica do Departamento de Cirurgia da Universidade Federal de São Paulo/Escola Paulista de Medicina - UNIFESP/EPM - São Paulo (SP) Brasil.
1. Médico Assistente da Disciplina de Cirurgia Torácica do Departamento de Cirurgia. Universidade Federal de São Paulo/Escola Paulista de Medicina - UNIFESP/EPM - São Paulo (SP) Brasil.
2. Professor Adjunto da Disciplina de Cirurgia Torácica do Departamento de Cirurgia. Universidade Federal de São Paulo/Escola Paulista de Medicina - UNIFESP/EPM - São Paulo (SP) Brasil.
3. Professor Adjunto Livre Docente da Disciplina de Cirurgia Torácica do Departamento de Cirurgia. Universidade Federal de São Paulo/Escola Paulista de Medicina - UNIFESP/EPM - São Paulo (SP) Brasil.
Endereço para correspondência: Altair da Silva Costa Júnior. Napoleão de Barros 715, 4º andar - Disciplina de cirurgia torácica, Vila Clementino, CEP 04024-002, São Paulo, SP, Brasil.
Tel 11 5576-4295. Fax 11 5572-0448. E-mail: cirurgiatoracica@uol.com.br
Suporte financeiro: Nenhum.
Recebido para publicação em 29/8/2007. Aprovado, após revisão, em 24/1/2008.




