ABSTRACT
Background: Tuberculosis continues to be a global health problem.
Objective: To evaluate an automated system designed to diagnose tuberculosis, comparing it to sputum microscopy and culture in Löwenstein-Jensen medium.
Method: A comparative study using 844 sputum samples, collected between September and December of 1999 at a reference center for tuberculosis in São Paulo, Brazil, to draw distinctions between the results obtained through the use of the automated system and those obtained through sputum microscopy and culture in Löwenstein-Jensen medium.
Results: Of the 844 samples evaluated, 27.1% tested positive for acid-fast bacilli, and 72.9% tested negative. In Löwenstein-Jensen culture, 34.7% were positive and 63% were negative, compared with 37.1% positivity and 56.9% negativity using the automated system. Sensitivity was 98.1% for the automated system and 91.9% for Löwenstein-Jensen culture. Specificity and positive predictive value were 100% for both methods. Negative predictive value was 98.9% for the automated system and 95.5% for Löwenstein-Jensen culture. The degree of accuracy was 99.3% for the automated system and 97% for Löwenstein-Jensen culture, and the Kappa was 0.99 for the automated system and 0.94 for Löwenstein-Jensen culture. The difference between the mean time to detection of mycobacteria using the automated system (10.5 days) and that found using Löwenstein-Jensen culture (34.7 days) was statistically significant.
Conclusion: The difference between the culture yield obtained using the automated system and that achieved with Löwenstein-Jensen culture was statistically significant. Mean time to detection of mycobacteria was significantly shorter with the automated system. The higher yield provided by this new system justifies its use in a reference center for tuberculosis in São Paulo
Keywords:
Mycobacterium tuberculosis. Diagnostic. Automated systems.
RESUMO
Introdução: A tuberculose permanece um problema de saúde pública mundial.
Objetivo: Avaliar um sistema de diagnóstico automatizado de tuberculose, comparando-o com baciloscopia e cultura em meio de Lowenstein-Jensen.
Método: Estudo comparativo entre os resultados obtidos no sistema automatizado, baciloscopia do escarro e em meio de Lowenstein-Jensen, em 844 amostras de escarro de setembro a dezembro de 1999, em centro de referência para tuberculose em São Paulo (SP).
Resultados: Das 844 amostras, 27,1% mostraram-se positivas para bacilo álcool-acidorresistente e 72,9% negativas. Nos cultivos em Lowenstein-Jensen, 34,7% foram positivas e 63% negativas; no sistema automatizado, 37,1% foram positivas e 56,9% negativas. Observou-se sensibilidade de 98,1% e 91.9% no sistema automatizado e no Lowenstein-Jensen, respectivamente. A especificidade e o valor preditivo positivo foram de 100% nos dois métodos. O valor preditivo negativo foi de 98,9% no sistema automatizado e de 95,5% no Lowenstein-Jensen. A acurácia foi de 99,3% no sistema automatizado e 97% no Lowenstein-Jensen e o Kappa de 0,99 no sistema automatizado e 0,94 no Lowenstein-Jensen. O tempo médio de detecção das micobactérias no sistema automatizado (10,5 dias) apresentou diferença estatística significativa quando comparado com o método de Lowenstein-Jensen (34,7 dias).
Conclusão: O rendimento da cultura com o sistema automatizado apresentou diferença estatística significativa quando comparado com o meio de Lowenstein-Jensen. O tempo médio de detecção das micobactérias foi significativamente reduzido no sistema automatizado. O rendimento do sistema automatizado justifica sua utilização em unidade de referência ambulatorial para a tuberculose em São Paulo.
Palavras-chave:
Mycobacterium tuberculosis. Diagnóstico. Sistemas automatizados.
INTRODUÇÃO
Durante muito tempo, predominou a idéia de que a tuberculose poderia ser controlada com os avanços obtidos nos meados do século recém-terminado(1). Apesar disso, observou-se a reemergência da tuberculose nos países desenvolvidos e um agravo da situação nos países em desenvolvimento, o que levou a Organização Mundial de Saúde a declarar, em seu relatório anual de abril de 1993, ser a tuberculose uma "emergência global"(2-4).
No Brasil, a tuberculose não pode ser considerada como uma doença reemergente, "pois nunca chegou a desaparecer", e "também não é doença emergente", pois o elevado nível da incidência é mantido. Pode-se considerar que o Brasil vive uma situação intermediária, entre os países desenvolvidos e a grave situação de alguns países do terceiro mundo, como, por exemplo, alguns países africanos. A expectativa que tínhamos na década de 1980 era de uma persistente e progressiva diminuição da doença, algo em torno de 4% a 6% ao ano. No início dos anos 1990, observou-se uma diminuição da taxa de decréscimo da tuberculose, com uma discreta piora da morbi-mortalidade, especialmente nos Estados onde foi maior a co-infecção tuberculose/vírus da imunodeficiência humana(4-7).
O Programa de Controle da Tuberculose no país tem por base a baciloscopia de escarro e a cultura, e admite o diagnóstico presuntivo, quando baseado nos achados clínicos e radiológicos característicos da doença. Os resultados obtidos, ainda que suficientes do ponto de vista epidemiológico, são inespecíficos, não permitindo, isoladamente, o diagnóstico conclusivo da tuberculose. Exames bacteriológicos mais complexos são necessários para a definição da etiologia(7-9).
O auxílio do diagnóstico laboratorial para a conclusão final, a mais importante contribuição é o achado do bacilo, seja pela inspeção direta (baciloscopia), ou por seu isolamento em cultivo (meios de cultura). Diferentes meios de cultura, tanto na forma sólida quanto na líquida, podem ser utilizados para cultivar as micobactérias. O meio de Lowenstein-Jensen (LJ) é o mais utilizado no país. O tempo médio de formação de colônias do M. tuberculosis é de quatro semanas, sendo esta sua grande desvantagem(7).
A questão do diagnóstico complica-se nos dias atuais com a simbiose entre a tuberculose e o vírus da imunodeficiência humana e a emergência de formas multirresistentes. Os recursos clássicos de diagnóstico da tuberculose não mais correspondem à nova demanda. Há a necessidade de recursos laboratoriais mais precisos e rápidos.
Com o avanço da tecnologia, surgiram métodos automatizados, como o BACTEC 460 System, um sistema radiométrico, utilizando meio de cultura líquido Middlebrook 7H9 com ácido palmítico marcado pelo carbono14. Este método possui a grande desvantagem de utilizar material radioativo. O sistema automatizado que mede o consumo de oxigênio (SA-O2) BACTEC-960®-MGIT TM utiliza o mesmo meio de cultura, mas com sensor fluorescente que reage à concentração de oxigênio do meio de cultura(9,10).
Este estudo tem como objetivo verificar o desempenho e a confiabilidade de um sistema de diagnóstico automatizado que mede o consumo de oxigênio para o diagnóstico laboratorial da tuberculose pulmonar, comparando-o com a baciloscopia e a cultura em LJ, com o objetivo de avaliar sua implantação em uma unidade de referência para a doença, na cidade de São Paulo (SP).
MÉTODO
Entre setembro e dezembro de 1999, 851 amostras de escarro de pacientes foram examinadas no Laboratório de Micobacteriologia do Instituto Clemente Ferreira, na cidade de São Paulo, pela baciloscopia para bacilos álcool-acidorresistentes segundo o método de Ziehl-Neelsen, semeadas no meio de LJ e no sistema SA-O2. Sete amostras foram excluídas por se tratar de micobactérias não tuberculosas.
Os exames das amostras de escarros foram realizados após descontaminação pelo método de Petroff(11), com a baciloscopia para bacilos álcool-acidorresistentes e a cultura em meio de LJ seguindo as orientações normativas do Ministério da Saúde(12).
A cultura no SA-O2 usou um meio líquido de Middlebrook 7H9 enriquecido com OADC e acrescido de PANTA. Os tubos de cultura contêm um sensor fluorescente de sais de rutênio, que emitem luz em baixas concentrações de oxigênio que, no caso, é consumido pelo bacilo. Uma célula fotoelétrica avalia, a cada hora, a luminescência dos tubos, registrando os resultados positivos quando da emissão de luz(9). Os resultados positivos foram confirmados por lâmina confeccionada a partir do sedimento de cultura, corado pelo método de Ziehl-Neelsen e lido pela microscopia óptica. Em seguida, procedeu-se à identificação das espécies, para exclusão das micobactérias não tuberculosas. Os resultados positivos no LJ e no SA-O2, além do exame baciloscópico, foram repicados em meio de LJ para posterior identificação da micobactéria, inclusive as culturas negativas no LJ e positivas no SA-O2, que foram positivas no repique utilizando meio de LJ.
As identificações das espécies micobacterianas foram processadas a partir de um cultivo recente em meio solidificado. Para isso, foi semeado 0,1ml de suspensão bacteriana em meio LJ contendo o ácido p-nitrobenzóico (PNB) e em outro contendo a hidrazida do ácido tiofeno-2-carboxílico (TCH). Os frascos foram incubados a 37oC por 28 dias. Após este tempo, foram interpretadas como sendo membros do M. tuberculosis complex, os inóculos que eram sensíveis em presença de ácido p-nitrobenzóico (não apresentavam crescimento) e resistentes à hidrazida do ácido tiofeno-2-carboxílico (apresentavam crescimento)(12).
Foram avaliadas estatisticamente, pelo teste de MacNemar, as taxas de contaminação entre os dois sistemas de cultura e analisados o tempo de crescimento do M. tuberculosis em cada um deles, com avaliação pelo teste t de Student. O estudo foi aprovado pela Comissão de Ética em Pesquisas com Seres Humanos do Instituto de Ciências Biomédicas da Universidade de São Paulo. Não houve conflito de interesses e o estudo foi realizado com recursos próprios da instituição.
RESULTADOS
No período estabelecido para o estudo foram coletadas 851 amostras de escarro, das quais 7 foram excluídas por se tratar de micobactérias não tuberculosas. Foram, portanto, analisadas 844 amostras, provenientes de 489 pacientes atendidos no Instituto Clemente Ferreira, a maioria realizando mais de um exame.
Os resultados da baciloscopia para bacilos álcool-acidorresistentes das 844 amostras de escarro foram: 229 positivos (27,1%) e 615 negativos (72,9%). Nos cultivos em LJ, 293 amostras foram positivas (34,7%) e 532 negativas (63,0%), enquanto que no SA-O2, as positivas foram 313 (37,1%) e as negativas, 480 (56,9%) (Tabela 1).

Em relação aos exames contaminados, 19 ocorreram no LJ (2,3%) e 51 no SA-O2 (6,0%), sendo maior neste último, o que foi estatisticamente significativo (p < 0,0001, teste de McNemar) (Tabela 2).
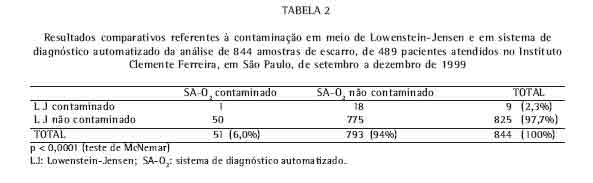
Dos 51 exames de SA-O2 contaminados, apenas 1 amostra se contaminou também no LJ. Da mesma maneira, dos 19 exames contaminados no LJ só a amostra citada acima se contaminou no SA-O2.
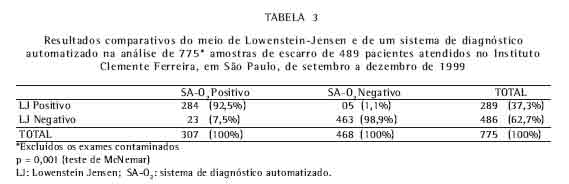
Dos resultados globais foram excluídos os exames contaminados, perfazendo um total de 775 amostras. A positividade do LJ foi de 289 e do SA-O2 de 307, havendo discordância em 28 espécimes, sendo 5 exames considerados negativos no SA-O2 e 23 no LJ. Portanto, o SA-O2 recuperou 18 amostras negativas no LJ (Tabela 3).
No caso de mais de um exame por paciente, o resultado foi considerado positivo quando pelo menos um deles foi positivo e, negativo, quando todos foram negativos. Com relação aos 161 pacientes diagnosticados a partir de cultura (158 positivos no SA-O2 e 3 positivos no LJ), as amostras foram tipificadas e os prontuários avaliados e concordantes quanto ao diagnostico de tuberculose pulmonar. As 3 amostras discordantes apresentaram baciloscopia positiva em 2 casos e negativa em 1 deles (Tabela 4).

Os cálculos estatísticos demonstraram sensibilidade de 98,1% no SA-O2 e de 91,9% no LJ. Já a especificidade e o valor preditivo positivo foram de 100% nos dois métodos. O valor preditivo negativo foi de 98,9% no SA-O2 e de 95,5% no LJ. A acurácia foi de 99,3% no SA-O2 e de 97% no LJ. A estatística de Kappa (grau de concordância corrigido) foi de 0,99 para o SA-O2 e de 0,94 no LJ.
Foram analisados os tempos de detecção para o M. tuberculosis nas amostras totais e por pacientes, com diferenças estatisticamente significativas entre os dois meios de cultura testados (p < 0,001). A média de crescimento no SA-O2 foi de 10,8 dias (90% variando entre 4 e 16) e 10,5 dias (90% entre 5 e 15), contra 37,9 dias (90% entre 29 e 45) e 34,7 dias (90% entre 28 e 42), no meio LJ, para todas as 844 amostras e para 438 amostras por pacientes, respectivamente. Do total de excluídos, 51 estavam com SA-O2 contaminados (Figura 1).

De uma maneira geral, foi observada uma redução no tempo de crescimento das micobactérias no meio SA-O2 para mais de um terço sobre o cultivo em meio sólido de LJ. Encontrou-se uma variação para o meio SA-O2 de 2 a 42 dias (limite do sistema) contra 23 a 60 dias (limite estabelecido) para o meio LJ.
DISCUSSÃO
Com a melhora acentuada do controle da doença nos países desenvolvidos, a qual ficou mais restrita aos países em desenvolvimento nos meados do século passado, predominou a concepção de que a tuberculose poderia ser controlada com os recursos existentes: métodos simples e econômicos de diagnóstico e potentes esquemas de quimioterapia(1).
Nas duas últimas décadas, entretanto, a situação da tuberculose sofreu alterações importantes, contribuindo, para isso, alguns fatores: aparecimento da pandemia da síndrome da imunodeficiência adquirida, com profundas interferências na epidemiologia da tuberculose; aumento da miséria e suas conseqüências (educação e habitação precárias, famílias numerosas, adensamentos comunitários, desnutrição alimentar, alcoolismo e doenças infecciosas associadas); falta de investimentos na saúde e deterioração da infra-estrutura de assistência, o que dificulta o acesso aos serviços (estes dois últimos fatores, mormente nos países do terceiro mundo); a tuberculose nos idosos, infectados na época de alta incidência da doença, aumentada nos países desenvolvidos pelo aumento da longevidade, constituindo fontes de infecção antes não encontradas; globalização da doença, a partir da migração dos povos pobres para os países ricos, os quais levam consigo, entre suas mazelas, a endemias e, entre estas, a tuberculose(2-7).
De certa forma, os avanços nos métodos diagnósticos ocorreram pela reemergência da tuberculose nos países avançados, que detêm os recursos técnicos para o seu desenvolvimento, forçados pela necessidade de resultados mais rápidos, com definição da espécie de micobactéria envolvida e seu perfil de resistência, especialmente frente à necessidade de procedimentos com a emergência da síndrome da imunodeficiência adquirida. A introdução destes métodos na prática rotineira, entretanto, deve passar sempre pela comprovação de sua aplicabilidade numa determinada região, especialmente nos países em desenvolvimento, definindo seu rendimento em relação aos padrões em uso e seu custo-benefício(13).
Assim, foi avaliado o rendimento do SA-O2, em uma unidade de referência, com alta demanda de pacientes com diagnósticos complicados e com problemas terapêuticos de diversas ordens, comparando-o aos métodos tradicionais em uso, à baciloscopia para bacilos álcool-acidorresistentes e à cultura em meio de LJ.
Uma das justificativas para o estudo é o número de exames realizados num curto período de tempo, tanto para diagnóstico como para controle do tratamento.
O SA-O2 representa um avanço nos meios rápidos de detecção do M. tuberculosis. Substitui o que tinha sido desenvolvido anteriormente, um sistema radiométrico conhecido como BACTEC 460 System (Becton Dickinson Md), de 1977, que detecta a micobactéria através de cultivo obtido em tempo estimado em 9 a 14 dias para amostras do complexo M. tuberculosis, e inferior a 7 dias para as micobactérias atípicas. Este instrumento mede a concentração de gás carbônico radioativo produzido pelas micobactérias em meio de Middlebrook 7H9, com ácido palmítico marcado com carbono14(14). Além do uso de material radioativo, utiliza agulhas para a inoculação.
O novo sistema SA-O2 tem como vantagens adicionais possuir metodologia não radioativa, avaliando o crescimento micobacteriano pela produção de oxigênio, medido através da fluorescência emitida pelo sal de rutênio, ser automatizado e medir concomitantemente mais do dobro de amostras que o sistema precedente.
No nosso país foram realizados trabalhos e estudos sobre o BACTEC 460 e isoladamente o Mycobactera Growth Indicator Tube(15-17), mas, até a realização deste estudo, não existiam trabalhos sobre a validação ou aplicabilidade do sistema SA-O2. Na literatura internacional, entretanto, já existem diversos relatos sobre o uso deste sistema, alguns comparativos com o meio LJ(18,19).
Os resultados positivos da análise das 844 amostras totais de escarro foram estatisticamente significativos, com percentuais de positividade de 35% para cultura no LJ e de 37% para o SA-O2, significância também encontrada nos exames individualizados por pacientes (n = 438), de 36% e 34%, respectivamente. Estes resultados são compatíveis com trabalhos internacionais, que documentam a mesma similitude(18,19) (Tabelas 1 e 2).
Das cinco amostras discordantes na Tabela 2 (amostragem total) em que o SA-O2 foi negativo e o LJ positivo, em 4 delas cresceram apenas poucas colônias no LJ, 2 tendo baciloscopia negativa e 2 positiva com poucos bacilos. Apenas 1 das 5 amostras apresentou baciloscopia (++) com crescimento em LJ (+) (de 20 a 100 colônias).
As 3 discordâncias que aparecem na Tabela 4 (uma amostra por paciente) apresentaram baciloscopia positiva em 2 casos e negativa em 1. Uma das amostras com baciloscopia positiva apresentou poucas colônias no LJ e a outra, com baciloscopia (++), teve o resultado em LJ de (+).
O sistema SA-O2, por utilizar um meio mais rico em nutrientes, tende a apresentar uma maior contaminação do que o meio sólido LJ, o que foi constatado no estudo atual, que encontrou uma taxa de contaminação significativamente maior no BM960 (BACTEC-960®-MGITTM) (5l/844) do que no LJ (19/844). Na literatura, há estudos, como o de Chien et al.(20), que não encontraram essa diferença. Essa discordância pode estar relacionada às características da clientela atendida na unidade, onde são freqüentes pacientes crônicos e diagnósticos demorados e com lesões extensas, o que deve facilitar a contaminação.
Outra importante constatação foi o tempo de detecção do M. tuberculosis, muito menor no SA-O2 (médias de 10,8 dias no total de amostras e de 10,5 dias quando individualizadas por paciente), menos que um terço do tempo no meio LJ (médias de 37,9 e 34,7 dias, respectivamente) (Figura 1).
Neste estudo, o rendimento da cultura do SA-O2, apresentou uma semelhança significativa com a do meio LJ. Em alguns casos, inclusive, apresentou sensibilidade e especificidade maiores. O tempo médio de detecção das micobactérias foi significativamente reduzido no SA-O2 em relação ao do LJ, adotado como padrão.
Podemos concluir, portanto, que o rendimento do SA-O2 justifica sua implantação nas condições operacionais de um laboratório de uma unidade de referência ambulatorial para a tuberculose, na cidade de São Paulo.
REFERÊNCIAS
1. Fox W, Mitchison DA. Quimioterapia de la tuberculosis. [Opas, publicacion cientifica, 310]. Washington: OPAS/OMS, 1975.
2. World Health Organization. TB: a global emergency. [WHO.TB 94.177] Geneve, 1994.
3. Chaisson RE, Benson CA. Tuberculosis and HIV infection. In: Rossman MD, MacGregor RR. Tuberculosis: clinical management and new challenges. New York: McGraw-Hill; 1996:Chap.14:223-238.
4. Rosemberg J. Tuberculose. Panorama global. Óbices para seu controle. Fortaleza: Secretaria de Estado da Saúde do Ceará; 1999.
5. Ruffino Netto A. Brasil e a tuberculose: doença emergente ou reemergente? Bol Pneumol Sanit 1997;5:3.
6. Campos HS, Fiuza de Melo FA. Efetividade do esquema 3 (3SZEEt/9EEt) no retratamento da tuberculose na rotina das Unidades de Saúde. Bol Pneumol Sanit 2000;8:7-14.
7. Ministério da Saúde/FUNASA/Centro de Referência Prof. Hélio Fraga - Sociedade Brasileira de Pneumologia e Tisiologia. Controle da tuberculose: uma proposta de integração ensino-serviço. 5a Rio de Janeiro; 2002: Chap. 5:61-96.
8. Fiuza de Melo FA, Afiune JB, Kritski AL, Seiscento M, Hijjar MA. Tuberculose. In: Veronesi R, Focaccia R, editores. Tratado de infectologia. São Paulo: Atheneu; 1996. p. 914-59.
9. Hanna BA. Diagnosis of tuberculosis by microbiologic techniques. In: Rom WN, Garay SM, editors. Tuberculosis. New York: Little, Brown and Co; 1995; Cap.11: 149-60.
10. Becton-Dickinson. Manual do usuário, BACTEC® MGITT; 1998.
11. Petroff AS. A new rapid method for the isolation and cultivation of tubercle bacille directly from sputum and fees. J Exp Med 1915.;21:38.
12. Ministério da Saúde/FUNASA/Centro de Referência Prof. Hélio Fraga. Manual de bacteriologia da tuberculose 2a. ed. Rio de Janeiro; 1994.
13. Kritski AL, Conde MB, Souza GRM. Tuberculose: do ambulatório à enfermaria. São Paulo: Atheneu; 1999.
14. Becton-Dickinson. Manual do usuário, BACTEC 460-System®;1985.
15. Palaci M, Ueki SY, Sato DN, Da Silva Telles MA, Curcio M, Silva EA. Evaluation of mycobacteria growth indicator tube for recovery and drug susceptibility testing of Mycobacterium tuberculosis isolates from respiratory specimens. J Clin Microbiol 1996;34:762-4.
16. Machado AMO. Avaliação de meio de cultura líquido BBL Mycobacteria Growth Indicator Tube (MGIT) em rotina de detecção de micobactérias em amostras de escarro de pacientes com suspeita de tuberculose pulmonar. [tese]. São Paulo: Escola Paulista de Medicina, Universidade Federal de São Paulo; 1998.
17. Martins FM, Caldas PC, Barreto AMW. Avaliação do antibiograma pelo método radiométrico para M. tuberculosis. Livro de Resumos do XX Congresso Brasileiro de Microbiologia. VIII Simpósio Brasileiro de Micobactérias 1999; 432.
18. Oplustil CP, Sinto SI, Marins M, Mendes CMF. Avaliação de um novo sistema para detecção de micobactérias: Mycobacterium Growth Indicator Tube (MGIT). J Bras Patol 1997;3:70-5.
19. Pfyffer GE, Welscher HM, Kissling P, Cieslak C, Casal MJ, Gutierrez J et al. Comparison of the Mycobacteria Growth Indicator Tube (MGIT) with radiometric and solid culture for recovery of acid-fast bacilli. J Clin Microbiol 1997;35:364-8.
20. Chien HP, Yu MC, Wu MH, Lin TP, Luh KT. Comparison of the Bactec MGIT 960 with Lowenstein-Jensen medium for recovery of mycobacteria from clinical specimen. Int J Tuberc Lung Dis 2000; 4:866-70.
* Trabalho realizado no Instituto Clemente Ferreira - Coordenadoria de Controle de Doenças - Secretaria de Estado da Saúde - São Paulo
** Título de Especialista pela Sociedade Brasileira de Pneumologia e Tisiologia
Endereço para correspondência: Elisabete Ap. Almeida. Rua da Consolação, 717. CEP: 01301-000, São Paulo, SP. Tel: 55 11 3257-0624. E-mail: bete.alme@ig.com.br
Recebido para publicação, em 18/5/04. Aprovado, após revisão em 20/1/05.








