ABSTRACT
Objective: Obstructive sleep apnea (OSA) is associated with an increased risk of mortality and cardiometabolic diseases. The STOP-Bang questionnaire is a tool to screen populations at risk of OSA and prioritize complementary studies. Our objective was to evaluate the clinical utility of this questionnaire in identifying patients at an increased risk of mortality after discharge in a cohort of hospitalized patients. Methods: This was a prospective cohort study involving consecutive patients admitted to an internal medicine unit between May and June of 2017 who were reevaluated three years after discharge. At baseline, we collected data on comorbidities (hypertension, obesity, diabetes, and fasting lipid profile) and calculated STOP-Bang scores, defining the risk of OSA (0-2 score, no risk; = 3 score, risk of OSA; and = 5 score, risk of moderate-to-severe OSA), which determined the study groups. We also recorded data regarding all-cause and cardiovascular mortality at the end of the follow-up period. Results: The sample comprised 435 patients. Of those, 352 (80.9%) and 182 (41.8%) had STOP-Bang scores = 3 and = 5, respectively. When compared with the group with STOP-Bang scores of 0-2, the two groups showed higher prevalences of obesity, hypertension, diabetes, and dyslipidemia. Multivariate analysis showed an independent association between cardiovascular mortality and STOP-Bang score = 5 (adjusted hazard ratio = 3.12 [95% CI, 1.39-7.03]; p = 0.01). Additionally, previous coronary heart disease was also associated with cardiovascular mortality. Conclusions: In this cohort of hospitalized patients, STOP-Bang scores = 5 were able to identify patients at an increased risk of cardiovascular mortality three years after discharge.
Keywords:
Sleep Apnea, Obstructive; Risk assessment; Surveys and questionnaires; Cardiovascular diseases/mortality.
RESUMO
Objetivo: A apneia obstrutiva do sono (AOS) está associada a um risco maior de mortalidade e doenças cardiometabólicas. O questionário STOP-Bang é uma ferramenta para rastrear populações em risco de AOS e assim priorizar estudos complementares. Nosso objetivo foi avaliar a utilidade clínica desse questionário na identificação de pacientes com risco aumentado de mortalidade após a alta em uma coorte de pacientes hospitalizados. Métodos: Estudo de coorte prospectivo com pacientes consecutivos internados em uma unidade de medicina interna entre maio e junho de 2017 que foram reavaliados três anos após a alta. No momento basal, coletamos dados sobre comorbidades (hipertensão, obesidade, diabetes e perfil lipídico em jejum) e calculamos as pontuações no STOP-Bang, definindo o risco de OSA (pontuação 0-2, sem risco; pontuação ≥ 3, risco de AOS; e pontuação ≥ 5, risco de AOS moderada a grave), que determinou os grupos de estudo. Também registramos dados sobre mortalidade por todas as causas e mortalidade cardiovascular ao final do período de acompanhamento. Resultados: Foram incluídos 435 pacientes. Desses, 352 (80,9%) e 182 (41,8%) apresentaram pontuações no STOP-Bang ≥ 3 e ≥ 5, respectivamente. Quando comparados com o grupo com pontuação no STOP-Bang de 0-2, os outros dois grupos apresentaram prevalências mais elevadas de obesidade, hipertensão, diabetes e dislipidemia. A análise multivariada mostrou uma associação independente entre mortalidade cardiovascular e pontuação no STOP-Bang ≥ 5 (razão de risco ajustada = 3,12 [IC95%, 1,39-7,03]; p = 0,01). Além disso, doença coronariana prévia também foi associada à mortalidade cardiovascular. Conclusões: Nesta coorte de pacientes hospitalizados, pontuações no STOP-Bang ≥ 5 foram capazes de identificar pacientes com risco aumentado de mortalidade cardiovascular três anos após a alta.
Palavras-chave:
Apneia obstrutiva do sono; Medição de risco; Inquéritos e questionários; Doenças cardiovasculares/mortalidade.
INTRODUÇÃO A apneia obstrutiva do sono (AOS) é uma condição prevalente, com risco aumentado de complicações cardiovasculares. Essa condição é prevalente e subdiagnosticada em alguns países, principalmente devido à falta de estudos do sono. Estudos anteriores relatam que 1 bilhão de pessoas está sob risco de AOS em todo o mundo.(1,2) No Chile, a população com risco de AOS é de 22%, e o risco de AOS moderada a grave é de cerca de 9%.(3) De acordo com a American Academy of Sleep Medicine, a AOS pode ser diagnosticada por meio de polissonografia ou de um monitor portátil de sono de três canais. Seu diagnóstico requer a presença de um índice de apneia-hipopneia (IAH) anormal (≥ 5 eventos/h) e a presença de pelo menos um dos seguintes sintomas: sonolência/fadiga, sono perturbado por engasgos ou sufocamento, ronco ou apneia testemunhada por um terceiro, ou comorbidades como hipertensão, doença arterial coronariana/acidente vascular cerebral, insuficiência cardíaca, diabetes e alterações de humor. O diagnóstico pode ser feito diretamente se IAH ≥ 15 eventos/h. A gravidade da AOS varia de leve (IAH entre 5-15 eventos/h), moderada (IAH entre 15-29 eventos/h) a grave (IAH ≥ 30 eventos/h).(4,5) A diferenciação entre AOS leve e AOS moderada a grave é relevante na prática clínica. AOS moderada a grave está associada a um risco aumentado de hipertensão, diabetes mellitus (DM), dislipidemia, obesidade e eventos cardiovasculares maiores — infarto agudo do miocárdio (IAM), doença coronariana (DC), acidente vascular cerebral, fibrilação atrial (FA) e mortalidade cardiovascular.(6)
Embora estudos do sono sejam escassos em alguns países, diferentes questionários clínicos estão disponíveis para identificar as populações com risco de AOS. Essa abordagem também é útil para definir populações com risco aumentado de comorbidades cardiovasculares, como pacientes hospitalizados. Além disso, por meio desses preditores clínicos, médicos clínicos também podem identificar populações com alta probabilidade pré-teste de AOS e, portanto, definir o estudo diagnóstico com melhor custo-benefício após a alta desses pacientes.
O questionário STOP-Bang é uma ferramenta de triagem clínica útil para pacientes com risco de AOS (pontuação limiar, ≥ 3) e de AOS moderada a grave (pontuação limiar, ≥ 5). A sensibilidade e a especificidade desse questionário são de 90% e 49%, respectivamente, usando uma ponto de corte ≥ 3 para identificar o risco de AOS. No entanto, usando uma ponto de corte ≥ 5, a sensibilidade e a especificidade para identificar o risco de AOS moderada a grave são de 96% e 25%, respectivamente. Dada a excelente sensibilidade, o questionário STOP-Bang tem sido proposto como ferramenta de triagem em diversos estudos epidemiológicos.(7,8)
A combinação de AOS com outras comorbidades cardiovasculares significativas incluídas no questionário STOP-Bang sugere que esse poderia ajudar a identificar a população com maior risco de mortalidade em médio prazo, especialmente em pacientes hospitalizados. O objetivo do presente estudo foi avaliar a associação entre o risco de AOS medido pelo questionário STOP-Bang e o risco de mortalidade por todas as causas e mortalidade cardiovascular em pacientes hospitalizados.
MÉTODOS Estudo de coorte prospectivo observacional seguindo as recomendações atuais de Strengthening the Reporting of Observational Studies in Epidemiology (STROBE).(9) Entre maio e junho de 2017, incluímos consecutivamente pacientes internados na unidade de medicina interna em um único centro de saúde universitário localizado na cidade de Los Ángeles, Chile.
A coorte incluída foi acompanhada prospectivamente até junho de 2020. Os pacientes admitidos por qualquer motivo médico foram selecionados para possível inclusão. Incluímos pacientes com > 18 anos de idade que deram consentimento informado por escrito. Foram excluídos os pacientes que não conseguiram preencher o questionário, aqueles submetidos a qualquer tipo de cirurgia durante a internação, os encaminhados para UTI, os considerados em final de vida por alguma comorbidade médica ou por perda de seguimento. O protocolo do estudo foi aprovado pelo Comitê de Ética em Pesquisa do IRB Servicio de Salud Bio-Bio (Protocolo nº 25, agosto de 2017). O estudo foi realizado de acordo com as diretrizes estabelecidas na Declaração de Helsinque e as boas práticas clínicas.
Foram coletados dados demográficos (sexo, idade [ser idoso foi definido como ter ≥ 65 anos de idade]), bem como histórico de tabagismo, consumo de álcool, comorbidades basais (hipertensão arterial, DM, DC e acidente vascular cerebral) e medicamentos atuais (para controle de dislipidemia, DM e hipertensão, assim como anticoagulantes). Os dados sobre comorbidades e o motivo da internação foram recuperados por autorrelato e prontuários médicos.
A exposição foi definida como o risco de OSA medido pela pontuação no STOP-Bang na admissão. O questionário foi avaliado de acordo com uma validação prévia na população do Chile.(7,8) Os sintomas relacionados ao sono (ronco, cansaço e apneia observada) foram registrados por autorrelato ou resposta do parceiro. A pressão arterial foi medida com esfigmomanômetro de mercúrio padrão no braço esquerdo após 10 min de repouso, de acordo com as diretrizes atuais da American Heart Association. (10) Peso e altura foram medidos após jejum noturno com os pacientes vestindo apenas roupas íntimas. O IMC foi calculado como peso (kg)/altura2 (m2), e a circunferência do pescoço foi medida com um medidor de fita plástica no nível da cricoide (pomo de Adão).
Uma amostra de sangue venoso foi coletada de todos os pacientes nas primeiras 48 h após a admissão. A amostra foi obtida pela manhã, após jejum noturno. Incluímos um perfil lipídico em jejum. Pacientes com perfil lipídico anormal foram classificados de acordo com o tipo de dislipidemia: dislipidemia LDL, hipertrigliceridemia, dislipidemia mista e dislipidemia HDL, de acordo com as recomendações atuais.(10) Utilizando os dados clínicos e laboratoriais, calculamos o risco cardiovascular através do escore de risco para Atherosclerotic Cardiovascular Disease (ASCVD, Doença Cardiovascular Aterosclerótica) do American College of Cardiology/American Heart Association(10) no momento basal na população de 34 a 79 anos.
O desfecho primário do presente estudo foi o risco de mortalidade por todas as causas após a alta em pacientes com uma pontuação no STOP-Bang ≥ 3 e naqueles com uma pontuação ≥ 5. Como referência, ambos os grupos foram comparados com pacientes com uma pontuação entre 0-2 no STOP-Bang após o acompanhamento. O desfecho secundário foi o risco de mortalidade cardiovascular nos mesmos grupos. Os dados de mortalidade foram obtidos pelo Servicio de Registro Civil e Identificación (www.registrocivil.cl) e categorizados como mortalidade por todas as causas ou mortalidade cardiovascular de acordo com a Classificação Internacional de Doenças, versão 9 (CID-9). Os detalhes da definição de mortalidade cardiovascular no presente estudo estão disponíveis no Quadro S1.
Dados individuais foram incluídos em um formulário de relato de caso e transferidos para planilha Excel. Os dados contínuos foram apresentados como médias e desvios-padrão e os dados categóricos como frequências. As diferenças intergrupos foram avaliadas usando o teste t de Student para dados contínuos e o teste do qui-quadrado ou o teste exato de Fisher para dados categóricos. ORs e respectivos IC95% também foram descritos.
A associação entre os grupos e o desfecho primário foi avaliada por meio da análise de sobrevida de Kaplan-Meier e do teste de log-rank (teste de Mantel-Cox). A taxa de incidência de mortalidade foi avaliada por razão de risco (RR) ajustada com regressão de riscos proporcionais de Cox. Como covariáveis, incluímos variáveis de confusão relacionadas a um risco aumentado de mortalidade cardiovascular não incluídas no questionário STOP-Bang (dislipidemia, história de tabagismo, consumo de álcool, DC anterior, pontuação de risco ASCVD e uso de medicamentos para dislipidemia, DM e hipertensão e medicamentos anticoagulantes no início do estudo). O nível de significância adotado foi p < 0,05. A análise estatística foi realizada com o pacote de software SPSS Statistics, versão 25.0 (IBM Corp., Armonk, NY, EUA).
RESULTADOS Um fluxograma do processo de seleção de pacientes é mostrado na Figura 1. Foram selecionados 510 pacientes, sendo 435 incluídos no estudo para análise posterior. A média de idade da coorte foi de 60,98 ± 17,10 anos, 199 (45,7%) dos pacientes eram considerados idosos, 233 (53,6%) eram do sexo masculino, 352 (80,9%) tinham uma pontuação no STOP-Bang ≥ 3, e 192 (44,1%) tiveram uma pontuação no STOP-Bang ≥ 5. Os escores de risco ASCVD em relação aos pacientes com pontuações no STOP-Bang de 0-2, ≥ 3 e ≥ 5, respectivamente, foram 10,1 ± 11,2%, 22,6 ± 16,3% e 24,6 ± 15,8%. Um resumo das características basais e dos motivos de admissão é mostrado na Tabela S1.
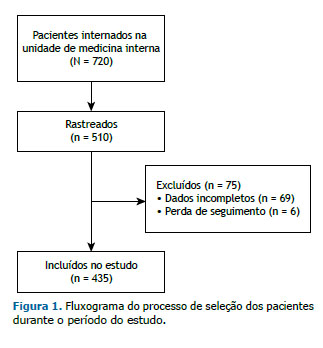
Um resumo das características clínicas e diferenças entre os grupos são mostrados na Tabela 1. A idade média no grupo com pontuação no STOP-Bang ≥ 3 (n = 352) foi de 62,0 ± 16,5 anos, 196 (55,7%) eram do sexo masculino, e o IMC médio foi de 33,9 ± 6,5 kg/m2. As prevalências de hipertensão, DM e dislipidemia foram 73,3%, 43,8% e 59,4%, respectivamente. Dos 209 pacientes com dislipidemia, 71 (34,0%) tinham hipertrigliceridemia; 60 (28,7%), dislipidemia LDL; e 78 (37,3%), dislipidemia mista. As razões cardiovasculares para a admissão foram insuficiência cardíaca, FA de início recente, angina instável e IAM.
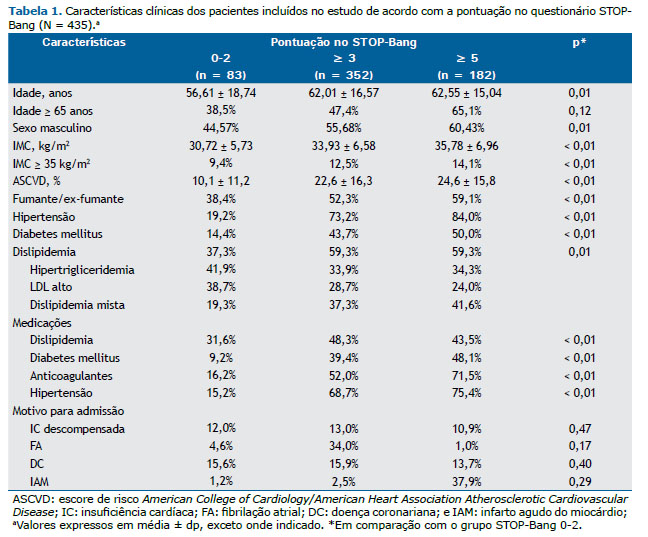
A média de idade no grupo com pontuação no STOP-Bang ≥ 5 (n = 182) foi de 62,55 ± 15,00, e o IMC médio foi de 35,78 ± 6,9 kg/m2. Dos 182 pacientes nesse grupo, as prevalências de hipertensão, DM e dislipidemia foram de 84,0%, 50,0% e 59,3%, respectivamente. Dos 108 pacientes com dislipidemia, 37 (34,3%) apresentavam hipertrigliceridemia; 26 (24,1%), dislipidemia LDL; e 45 (41,7%), dislipidemia mista. Os motivos de internação nesse grupo foram insuficiência cardíaca, em 10,9%; FA de início recente, em 1,1%; angina instável, em 13,7%; e IAM, em 37,9%.
Em comparação com o grupo controle (pontuação no STOP-Bang de 0-2), aqueles com pontuação ≥ 3 e ≥ 5 apresentaram perfil clínico semelhante, sem diferenças estatisticamente significantes. Um maior número de pacientes em ambos os grupos de estudo eram idosos (p = 0,09) e do sexo masculino (p = 0,01). Além disso, os dois grupos de estudo apresentaram maiores prevalências de comorbidades (IMC ≥ 35 kg/m2, hipertensão, DM e dislipidemia; p < 0,01 para todos). Embora não tenham sido encontradas diferenças em relação aos eventos cardiovasculares na admissão entre os grupos controle e estudo (Tabela 1), houve um aumento significativo do risco de mortalidade por todas as causas e mortalidade cardiovascular (p = 0,04 e p = 0,01, respectivamente) no grupo com pontuação no STOP-Bang ≥ 5 (Tabela 2).
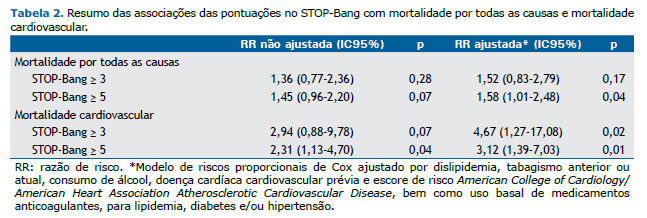
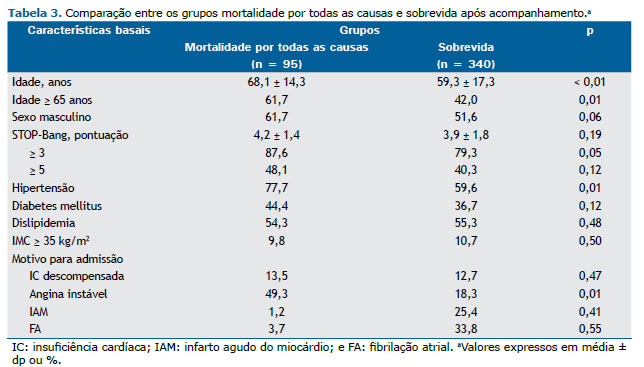
Após 36 meses de acompanhamento, 95 pacientes morreram, sendo que 34 deles faleceram por eventos cardiovasculares. As características clínicas daqueles que morreram ou sobreviveram são apresentadas nas Tabelas 3 e 4. Na análise não ajustada, uma pontuação no STOP-Bang ≥ 3 não demonstrou associações significativas com mortalidade por todas as causas (RR = 1,36 [IC95%: 0,77-2,36]; p = 0,28) ou mortalidade cardiovascular (RR = 2,94 [IC95%: 0,88-9,78]; p = 0,07). No entanto, a análise ajustada mostrou uma associação significativa com mortalidade cardiovascular (RR = 4,67 [IC95%: 1,27-17,08]; p = 0,02).
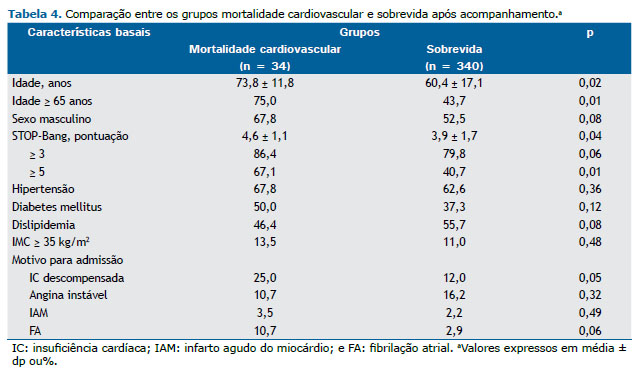
Na análise não ajustada, uma pontuação no STOP-Bang ≥ 5 não teve associação com mortalidade por todas as causas de (RR = 1,45 [IC95%: 0,96-2,20]; p = 0,17). No entanto, a análise ajustada mostrou uma associação significativa (RR = 1,58 [IC95%: 1,01-2,48]; p = 0,04). Com relação à mortalidade cardiovascular, tanto as análises não ajustadas quanto as ajustadas mostraram associações significativas com uma pontuação no STOP-Bang ≥ 5: RR = 2,31 (IC95%: 1,13-4,70); p = 0,02; e RR = 3,12 (IC95%: 1,39-7,03); p = 0,01, respectivamente (Tabela 2 e Figura 2). O resumo das análises não ajustadas e ajustadas é mostrado na Tabela 2. Além disso, DC prévia basal também foi associada à mortalidade cardiovascular no grupo STOP-Bang ≥ 5 (RR = 2,34 [IC95%: 1,04-5,26]; p = 0,04).
DISCUSSÃO Os principais achados do presente estudo foram que o questionário STOP-Bang foi capaz de identificar o risco cardiovascular em pacientes hospitalizados até 36 meses de seguimento; que, após análises ajustadas, as variáveis independentes associadas a um maior risco de mortalidade cardiovascular foram pontuação no STOP-Bang ≥ 3 e DC basal; e que a pontuação no STOP-Bang ≥ 5 estava associada a um maior risco de mortalidade cardiovascular e mortalidade por todas as causas.
Pacientes hospitalizados apresentam níveis de risco diferentes dos na população geral. Em nosso estudo, formulamos a hipótese de que o questionário STOP-Bang poderia identificar populações com risco aumentado de mortalidade cardiovascular para priorizar estudos adicionais do sono. Além disso, encontramos um aumento da prevalência de pacientes com risco de AOS quando comparamos os resultados de estudos anteriores de base hospitalar.(11-13) Sharma et al. (11) relataram uma prevalência de AOS em 84% dos pacientes obesos hospitalizados (IMC ≥ 30 kg/m2).(11) Goring & Collop(12) relataram uma prevalência de AOS diagnosticada por polissonografia de 77% em pacientes hospitalizados. Identificar pacientes hospitalizados com risco de AOS é uma questão importante, pois eles apresentam eventos com resposta mais rápida. Portanto, o uso de questionários validados que indicam um maior risco de AOS é uma intervenção útil para reduzir complicações.(13) O presente estudo encontrou um risco aumentado para AOS (80%) em nossa amostra, e 40% dos pacientes tiveram uma pontuação no STOP-Bang ≥ 5. Essa alta prevalência se deve à idade avançada, obesidade e hipertensão, que são comuns em pacientes hospitalizados. Além disso, após um período de acompanhamento de três anos, esse aumento da prevalência foi associado a um aumento do risco de mortalidade cardiovascular.
O questionário STOP-Bang foi inicialmente desenvolvido por pesquisadores da área de anestesiologia.(14) Estudos anteriores avaliaram sua acurácia em diferentes práticas clínicas, como em centros de estudos do sono ou em ambientes perioperatórios.(15,16) Esse questionário é uma ferramenta de uso fácil para rastrear populações com risco de OSA. Além disso, o questionário STOP-Bang tem se mostrado uma boa ferramenta de triagem para identificar indivíduos com hipertensão ou DM com risco de AOS.(17-19)
Dados sobre o uso do questionário STOP-Bang em pacientes hospitalizados são escassos, e a utilidade clínica desse questionário nessa população não é clara. Anteriormente, analisamos as relações transversais dos escores do questionário STOP-Bang com eventos cardiovasculares (desfechos compostos incluíram eventos cardiovasculares adversos maiores, mortalidade cardiovascular, síndrome coronariana aguda e insuficiência cardíaca descompensada) durante os primeiros 30 dias de hospitalização e com risco cardiovascular usando a calculadora de pontuação de risco ASCVD. De acordo com o escore de risco ASCVD, descobrimos que o risco cardiovascular em pacientes com pontuação no STOP-Bang ≥ 3 foi de 24,3%, enquanto esse foi de 10,9% naqueles com pontuação no STOP-Bang de 0-2.(20)
No presente estudo, incluímos uma única coorte de uma população subestudada (indivíduos hospitalizados internados em uma unidade de medicina interna) acompanhados por 36 meses para determinar a utilidade clínica do questionário STOP-Bang na identificação do risco de mortalidade por todas as causas e mortalidade cardiovascular. Nossa hipótese foi de que as populações com risco de AOS teriam um risco aumentado de mortalidade cardiovascular, principalmente devido a eventos cardiovasculares definidos na CID-9. Predefinimos a inclusão de pacientes hospitalizados com doença leve a moderada para descartar aqueles com doença aguda grave, que podem apresentar risco aumentado de morbidade e sequelas. Nossos resultados fornecem novos dados sobre pacientes hospitalizados. Estudamos a utilidade clínica do questionário STOP-Bang em uma população específica com risco de AOS em geral ou AOS moderada a grave. Nossos resultados demonstraram diferenças clínicas entre pacientes com risco de AOS e aqueles sem esse risco. Além disso, após a análise de fatores de confusão, DC prévia basal também foi associada à mortalidade cardiovascular.
Também encontramos um aumento da prevalência de doença cardiometabólica em pacientes com risco de AOS. Em primeiro lugar, a prevalência de hipertensão variou entre 73% e 84%, superior à prevalência relatada em outros estudos.(21) No entanto, a maioria desses estudos foi realizada em centros de estudos do sono, não com pacientes hospitalizados. Em segundo lugar, a prevalência de DM variou entre 44% e 50%. Pataka et al.(17) relataram uma prevalência de DM de 57% em pacientes com AOS grave; a sensibilidade do questionário STOP-Bang nos pacientes com DM foi de 81% para AOS leve e de 95% para AOS grave. Naquele estudo,(17) a prevalência de dislipidemia foi de 14%, contra 59% em nosso estudo. Um risco aumentado de dislipidemia foi previamente relatado por Chou et al.(22) em pacientes com AOS (61,1%). Em outro estudo,(23) a prevalência de dislipidemia foi de 26% na população geral. De acordo com dados do European Sleep Apnea Database,(24) a prevalência de hiperlipidemia foi de 26% e 15% em pacientes com e sem AOS, respectivamente, e esse aumento foi independente após a análise de fatores de confusão. No entanto, esses estudos foram realizados em cenários ambulatoriais, como estudos de base populacional ou em centros de estudos do sono. Por fim, em relação aos motivos cardiovasculares de admissão, a alta de pacientes com insuficiência cardíaca e AOS foi independentemente associada a um aumento do risco de mortalidade após 6-12 meses de seguimento (RR = 1,53).(25)
Os pontos fortes do presente estudo são o seu desenho prospectivo, incluindo pacientes latino-americanos consecutivos admitidos por qualquer motivo médico em um serviço de medicina interna e o fato de que o preenchimento do questionário STOP-Bang e a avaliação inicial foram realizados nas primeiras 48 h após admissão.
O estudo também tem limitações. Os pacientes incluídos não foram submetidos a estudos do sono para confirmação ou exclusão de AOS. No entanto, este estudo foi desenhado para avaliar o risco de mortalidade por meio do questionário STOP-Bang, e esses dados podem ser usados para melhorar a identificação de pacientes hospitalizados com risco aumentado de AOS e, portanto, de piores desfechos, o que pode permitir a incorporação de várias intervenções para reduzir esse risco.
Em conclusão, nesta coorte de pacientes hospitalizados em uma unidade de medicina interna, o questionário STOP-Bang foi capaz de identificar aqueles com maior risco de mortalidade. Uma pontuação no STOP-Bang ≥ 5 é uma ferramenta fácil e útil para identificar pacientes com risco de mortalidade cardiovascular e mortalidade por todas as causas em um período de acompanhamento de três anos.
CONTRIBUIÇÃO DOS AUTORES GL: desenho do estudo e autor responsável pelo estudo. JC, AS, BS, FR e GV: extração de dados. AS, GL e GV: análise de dados. GL, JC, GV, FR, AO e CN: interpretação dos resultados. GL, GV, JC, AO e CN: redação do manuscrito. GL, FR, AS, BS, AO e CN: revisão e edição do manuscrito. Todos os autores aprovaram a versão final do manuscrito.
REFERÊNCIAS 1. Lee W, Nagubadi S, Kryger MH, Mokhlesi B. Epidemiology of Obstructive Sleep Apnea: a Population-based Perspective. Expert Rev Respir Med. 2008;2(3):349-364. https://doi.org/10.1586/17476348.2.3.349
2. Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687-698. https://doi.org/10.1016/S2213-2600(19)30198-5
3. Carrillo J, Vargas C, Cisternas A, Olivares-Tirado P. Obstructive sleep apnea: findings from the Chilean National Health Survey 2010 Eur Resp J. 2017;50:PA1190. https://doi.org/10.1183/1393003.congress-2017.PA1190
4. Qaseem A, Dallas P, Owens DK, Starkey M, Holty JE, Shekelle P, et al. Diagnosis of obstructive sleep apnea in adults: a clinical practice guideline from theAmerican College of Physicians. Ann Intern Med. 2014;161(3):210-220. https://doi.org/10.7326/M12-3187
5. Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, et al. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13(3):479-504. https://doi.org/10.5664/jcsm.6506
6. Labarca G, Cruz NR, Descalzi F. Multisystemic involvement in obstructive sleep apnea [Article in Spanish]. Rev Med Chil. 2014;142(6):748-757. https://doi.org/10.4067/S0034-98872014000600009
7. Saldías Peñafiel F, Gassmann Poniachik J, Canelo López A, Uribe Monasterio J, Díaz Patiño O. Accuracy of sleep questionnaires for obstructive sleep apnea syndrome screening [Article in Spanish]. Rev Med Chil. 2018;146(10):1123-1134. https://doi.org/10.4067/S0034-98872018001001123
8. Labarca G, Dreyse J, Salas C, Gaete MI, Jorquera J. Performance of instruments aimed at detecting obstructive sleep apnea syndrome among individuals in Chile. J Bras Pneumol. 2019;46(1):e20190015. https://doi.org/10.1590/1806-3713/e20190015
9. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296. https://doi.org/10.1371/journal.pmed.0040296
10. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in J Am Coll Cardiol. 2014 Jul 1;63(25 Pt B):3024-3025] [published correction appears in J Am Coll Cardiol. 2015 Dec 22;66(24):2812]. J Am Coll Cardiol. 2014;63(25 Pt B):2889-2934. https://doi.org/10.1016/j.jacc.2013.11.002
11. Sharma S, Mather PJ, Efird JT, Kahn D, Shiue KY, Cheema M, et al. Obstructive Sleep Apnea in Obese Hospitalized Patients: A Single Center Experience. J Clin Sleep Med. 2015;11(7):717-723. https://doi.org/10.5664/jcsm.4842
12. Goring K, Collop N. Sleep disordered breathing in hospitalized patients. J Clin Sleep Med. 2008;4(2):105-110. https://doi.org/10.5664/jcsm.27125
13. Sharma S, Chowdhury A, Tang L, Willes L, Glynn B, Quan SF. Hospitalized Patients at High Risk for Obstructive Sleep Apnea Have More Rapid Response System Events and Intervention Is Associated with Reduced Events. PLoS One. 2016;11(5):e0153790. https://doi.org/10.1371/journal.pone.0153790
14. Nagappa M, Liao P, Wong J, Auckley D, Ramachandran SK, Memtsoudis S, et al. Validation of the STOP-Bang Questionnaire as a Screening Tool for Obstructive Sleep Apnea among Different Populations: A Systematic Review and Meta-Analysis. PLoS One. 2015;10(12):e0143697. https://doi.org/10.1371/journal.pone.0143697
15. Nagappa M, Patra J, Wong J, Subramani Y, Singh M, Ho G, et al. Association of STOP-Bang Questionnaire as a Screening Tool for Sleep Apnea and Postoperative Complications: A Systematic Review and Bayesian Meta-analysis of Prospective and Retrospective Cohort Studies. Anesth Analg. 2017;125(4):1301-1308. https://doi.org/10.1213/ANE.0000000000002344
16. Boynton G, Vahabzadeh A, Hammoud S, Ruzicka DL, Chervin RD. Validation of the STOP-BANG Questionnaire among Patients Referred for Suspected Obstructive Sleep Apnea. J Sleep Disord Treat Care. 2013;2(4):10.4172/2325-9639.1000121.
17. Pataka A, Kalamaras G, Daskalopoulou E, Argyropoulou P. Sleep questionnaires for the screening of obstructive sleep apnea in patients with type 2 diabetes mellitus compared with non-diabetic patients. J Diabetes. 2019;11(3):214-222. https://doi.org/10.1111/1753-0407.12835
18. Schiavone M, Ernst G, Blanco M, Avaca H, Acosta AL, Nosetto D, et al. Performance of questionnaires aimed at detecting sleep disorders in patients attending a hypertension center. Clin Exp Hypertens. 2019;41(7):687-691. https://doi.org/10.1080/10641963.2018.1539095
19. Westlake K, Plihalova A, Pretl M, Lattova Z, Polak J. Screening for obstructive sleep apnea syndrome in patients with type 2 diabetes mellitus: a prospective study on sensitivity of Berlin and STOP-Bang questionnaires. Sleep Med. 2016;26:71-76. https://doi.org/10.1016/j.sleep.2016.07.009
20. Labarca G, Valdivia G, Oñate A, Navarrete C, Araya J, Fernandez-Bussy I, et al. Prevalence of STOP BANG questionnaire and association with major cardiovascular events in hospitalized population: is it enough with currently used cardiovascular risk measurements?. Sleep Med. 2019;61:82-87. https://doi.org/10.1016/j.sleep.2019.02.019
21. Salas C, Dreyse J, Contreras A, Nazar G, Astorquiza C, Cabezon R, et al. Differences in patients derived from otolaryngology and other specialties with sleep apnea. J Otolaryngol Head Neck Surg. 2019;48(1):53. https://doi.org/10.1186/s40463-019-0373-4
22. Chou YT, Chuang LP, Li HY, Fu JY, Lin SW, Yang CT, et al. Hyperlipidaemia in patients with sleep-related breathing disorders: prevalence & risk factors. Indian J Med Res. 2010;131:121-125.
23. Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310-318. https://doi.org/10.1016/S2213-2600(15)00043-0
24. Gunduz C, Basoglu OK, Hedner J, Bonsignore MR, Hein H, Staats R, et al. Hyperlipidaemia prevalence and cholesterol control in obstructive sleep apnoea: Data from the European sleep apnea database (ESADA). J Intern Med. 2019;286(6):676-688. https://doi.org/10.1111/joim.12952
25. Khayat R, Jarjoura D, Porter K, Sow A, Wannemacher J, Dohar R, et al. Sleep disordered breathing and post-discharge mortality in patients with acute heart failure. Eur Heart J. 2015;36(23):1463-1469. https://doi.org/10.1093/eurheartj/ehu522









